CELL CYCLE AND DIFFERENTIATION IN GIARDIA LAMBLIA
Transcript of CELL CYCLE AND DIFFERENTIATION IN GIARDIA LAMBLIA

Department of Microbiology, Tumor- and Cell BiologyKarolinska Institutet, Stockholm, Sweden
CELL CYCLE AND DIFFERENTIATIONIN GIARDIA LAMBLIA
David S. Reiner
Stockholm 2008

Published by Karolinska Institutet.Printed by Larserics Digital Print AB, Sundbyberg, Stockholm, Sweden.© David S. Reiner, 2008ISBN: 978-91-7409-026-0

Järn giver skärpa åt järn; så skärper den ena människan den andra.
Ordspråksboken 27:17

ABSTRACT
Giardia lamblia is the major cause of waterborne diarrhea worldwide. Giardiasis is initiated byingestion of cysts, which after passing through the stomach are triggered to excyst. Excystation,or awakening from dormancy, is central to successful colonization of the host. An investigationof the role of calcium signaling throughout excystation was initiated to study the cellularregulation of this special differentiation. Calcium signaling was most crucial during lateexcystation where the excyzoite emerges. A calcium pump inhibitor, thapsigargin, inhibitedexcystation, calcium signaling and localized to a calcium storage compartment in cysts.Inhibitors of the calcium signaling protein calmodulin blocked excystation and calmodulinlocalized to Giardia’s basal bodies suggesting that the basal bodies are Giardia’s cellularcontrol center. Basal bodies were isolated and 310 proteins identified using proteomics.Functional orthologs of these proteins were identified bioinformatically and used to build anetwork model. Differentiation-specific nodes were identified in the network usingtranscriptional data from the Giardia lifecycle. The model correctly predicts that calmodulin isinvolved in cytoskeletal remodeling and this was verified by affinity purifying 10 calmodulin-specific binding proteins.
For cysts to survive in nature and the pass through the stomach successfully theyneed a protective wall. A study was undertaken to look for new cyst wall proteins. One cystwall protein identified was identified by SAGE and localized to the cyst body. This new cystwall protein was found to be an invariant cysteine-rich Type 1 membrane protein and amember of a larger cysteine-rich family. This new family of novel cysteine-rich Giardiaproteins was shown bioinformatically to have homologs in two other cyst-forming protozoans.
The initiation of differentiation is associated with cell cycle arrest in many cells.Giardia differentiates and forms cysts by arresting from the cell cycle and encysting. We lookedat the role of the cell cycle in Giardia during encystation. We developed for the first time amethod of synchronizing Giardia for use to determine where the encystation restriction point isin the cell cycle. We found using encystation-specific organelle biogenesis as a marker, that itwas late in G2. In addition we used quantitative real-time PCR to determine the periodic cellcycle regulation of histones. Cyclin B is normally up-regulated in the late G2 stage of the cellcycle and promotes G2/M transition. We phylogenomically identified a Giardia cyclin B andfound that expression gradually increased reaching a maximum at 3 h corresponding to G2, anddecreased again with entry into mitosis after 4 h. We also identified bioinformatically 217 cellcycle orthologs and studies are in progress to verify these using synchronized populations andGiardia microarrays.

LIST OF PUBLICATIONS
I. David S. Reiner, Michael L. Hetsko, J. Gary Meszaros, Chin-Hung Sun,Hilary G. Morrison, Laurence L. Brunton, and Frances D. Gillin:“Calcium signaling in excystation of the early diverging eukaryote, Giardialamblia”J. Biol. Chem. (2003) 278: 2533–2540.
II. Barbara J. Davids*, David S. Reiner*, Shanda R. Birkeland, Sarah P.Preheim, Michael J. Cipriano, Andrew G. McArthur, and Frances D. Gillin:“A New Family of Giardial Cysteine-Rich Non-VSP Protein Genes and aNovel Cyst Protein”PLoS ONE. (2006) 1: e44
III. David S. Reiner, Johan Ankarklev, Karin Troell, Daniel Palm,Rolf Bernander, Frances D. Gillin, Jan O. Andersson, and Staffan G. Svärd:“Synchronisation of Giardia lamblia: Identification of cell cycle stage-specific genes and a differentiation restriction point”Int. J. Parasitol. (2008) In press.
IV. Tineke Lauwaet, Michael Baitaluk*, David S. Reiner*, Edwin P. Romijn,Catherine C. L. Wong, Hanna Skarin, Barbara J. Davids, Shanda R.Birkeland, Michael J. Cipriano, Daniel Palm, Sarah P. Preheim, AmarnathGupta, Staffan G. Svärd, Andrew G. McArthur, John R. Yates 3rd, AnimeshRay, and Frances D. Gillin:“Unraveling the role of Giardia basal bodies in differentiation throughproteome, transcriptome and interactome analyses”Submitted.
• These two authors contributed equally to the paper All published papers were reproduced with permission from the publisher.

TABLE OF CONTENTS
1 INTRODUCTION 1
2 THE DISEASE 2
3 THE PARASITE 3
3.1 Plasma membrane proteins 3
3.2 Cytoskeleton 4 3.2.1 Flagella 5 3.2.2 Basal bodies 5 3.2.3 Ventral disk 6 3.2.4 Median body 6
4 DIFFERENTIATION 7
4.1 Encystation 7 4.1.1 Encystation stimuli 8 4.1.2 Cyst wall biogenesis 8
4.2 Cyst 94.3 Excystation 9
5 CELL CYCLE 10
5.1 Growth 10
5.2 Ploidy 11
6 COMPUTATIONAL BIOLOGY 12
6.1 Genome 12
6.2 Transcriptome 13
6.3 Proteomics 14
6.4 Interolog network 15

7 AIMS OF THE PRESENT STUDY 16
7.1 Paper I 17
7.2 Paper II 18
7.3 Paper III 21
7.4 Paper IV 23
8 CONCLUSIONS 26
9 ACKNOWLEDGEMENTS 28
10 REFERENCES 31

LIST OF ABBREVIATIONS
[Ca2+]i intracellular calcium
2-DE two-Dimensional gel Electrophoresis
BLAST Basic Local Alignment Search Tool
CaM CalModulin
CWP Cyst Wall Protein
ERK1 Extracellular signal-Regulated Kinase 1
ESV Encystation-Specific Vesicle(s)
GO Gene Ontology
HCMp High Cysteine Membrane protein
HCNCp High Cysteine Non-variant Cyst protein
MALDI Matrix-Assisted Laser Desorption/Ionization
MTOC MicroTubule Organizing Center
MudPIT Multidimensional Protein Identification Technology
NCBI National Center for Biotechnology Information
NIH National Institutes of Health, Bethesda, Maryland USA
PFR ParaFlagellar dense Rod
PP2A Protein Phosphatase 2A
QPCR real-time Quantitative PCR
SAGE Serial Analysis of Gene Expression
SALP1 Striated fiber Assemblin-Like Protein 1
SERCA Sarco Endoplasmic Reticulum Ca2+ATPase
TG Thapsigargin
TMHMM TransMembrane Hidden Markov Model software
VSP Variant Surface Protein(s)

1
1 INTRODUCTION
My first exposure to parasites was in Seoul, South Korea. Or at least that is what I toldmy interviewers when applying for a technical position working with Giardia lambliaand Entamoeba histolytica in the Laboratory of Parasite Growth and Differentiation at theNIH. After teaching a year of conversational English I had just returned to the UnitedStates with a sensitive and testy gut, months after an undiagnosed intestinal infection.This phenomenom, I now know is called irritable bowel syndrome, and is seen after viral,bacterial and parasitic outbreaks [1, 2]. Although I knew better, I had accepted (forsocial reasons), a gift of food from a student, which I promptly consumed in front of theclass. Now 33 years later, I still vividly remember, how sick and ugly my very own“intestinal outbreak” from that innocent gift was. Looking back now, it is quite clear thatone incident determined my research future. Looking back, I wouldn’t want it any otherway. A majority of the diseases of the developing world, specifically, viruses, bacteriaand parasites are diseases transmitted in the air, food and water, not the diseases ofoverconsumption and inactivity seen in the “developed world”. Besides poverty, thesediseases exist for the most part, because of our inability to effectively counteract thecountless “tricks and treats” of infectious organisms.
By the time I gladly accepted a once-in-a-lifetime opportunity, to study in the Departmentof Tumor and Infection Biology, I was already familiar with some infectious diseases, butdid not understand at all the connection to tumor biology. I have since (hopefully)learned, that the ability to differentiate, is what makes tumors and microbes, dangerous inthe first place. They use the cell cycle (growth) to increase population density, and stackthe odds in their favor, for continuing transmission and re-infection. Like a lazy relative,that visits but never leaves (without persuasion), they are masters of their own micro-universes, by fulfilling their needs, with minimal cellular effort. Intestinal parasites likeEntamoeba and Giardia, are environmental survival specialists, equally adept in a coolmountain stream or the warm-dark-moist-anaerobic shelter of an unsuspecting host. Theonly barrier between the outside world and the interior of the human body, is a singleepithelial cell layer [1]. By differentiating, Giardia thrives in this forbidding milieu,because of its unique morphology and mastery at exiting and re-entering its own cellcycle. This thesis examines specific aspects of Giardia’s transduction, survival andresponses of known host stimuli, and proposes a network model of the cellular controllerof those responses.
Dr. Louis Diamond, Section Chief of the Laboratory of Parasitic Diseases, from 1959-1994. Developed TYI-S-33 medium used growing Giardia, Entamoeba, Trichomonas and Spironucleus. Was the first of my two interviewers.

2
2 THE DISEASE
The parasitic protozoan Giardia lamblia (syn. Giardia intestinalis, Giardia duodenalis)is a leading cause of waterborne diarrhea worldwide [3]. Foodborne infections are lesscommon. Fecal contamination in nursing homes and day care centers also leads toinfection. In young children, acute giardiasis is responsible for rapid electrolyte loss,while chronic diarrhea causes malabsorption, often resulting in failure to thrive. Acutedisease typically begins 1-2 weeks after infection and approximately half of the peopleinfected with Giardia remain asymptomatic. Giardia‘s pathophysiology is poorlyunderstood, as it does not invade or cause much inflammation. Typically infection withenteric pathogens induces the expression of chemokines and cytokines in the intestinalepithelium of the host. But a gene expression study analyzing several cytokines inhuman intestinal cells after a 5 hours infection with Giardia lamblia [1], did not revealhigh levels of (IL)-8 cytokine release, which usually reflects inflammation duringbacterial infections [2]. The low levels of (IL)-8 seen could help explain the knownlack of inflammation, or alternatively, Giardia actively downregulates theinflammatory response [3]. Although many diseases mechanisms have been proposed,no conventional toxin or virulence factor has been identified. Therefore understandingthe mechanisms of attachment, growth, colonization and differentiation is vitallyimportant.

3
3 THE PARASITE
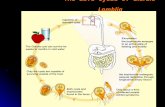
The parasite Giardia lamblia has two distinct and specialized forms, the trophozoite andthe cyst. The trophozoite has a characteristic half pear-shaped body, 12–15 mm long and5–9 mm wide with four pairs of flagella which exit the parasite at specific positions [4].After staining, it presents as a face, with the nuclei being the eyes, the anterior flagellarrods the eyebrows and the median body appearing as a “frown. The prominent feature ofthe ventral surface is the attachment disk, used for anchoring to substrates, both naturaland artificial. The infectious form of Giardia is the environmentally hardy cyst, with only10 cysts required to transmit the disease, as first demonstrated in a Texas prison“volunteer” population in 1954 [5]. After ingestion and exposure to stomach acid, cystsare triggered to differentiate or excyst after passage into the small intestine. The emergentparasite or “excyzoite” quickly attaches to the host epithelium, using the specializedventral disk in order to resist peristaltic elimination. Subsets of trophozoite populationsexposed to specific upper intestinal factors are induced to encyst. These “encyzoites”develop novel organelles, the encystation-specific vesicles (ESV), which carry thecomponents for forming the wall of the infectious cyst thus completing the lifecycle.
Giardia lamblia has been suggested to comprise a species complex, with sevenmorphologically identical but genetically distinct assemblages. The role of zoonotictransmission is still uncertain, as is the connection between the severity of infection anddifferent assemblages [6]. Giardia lamblia is the only species recovered from humans,and although apparently identical in morphology, there is a large genetic divergence,leading to the proposition that Giardia may be a species complex. The genotypes isolatedinclude the original A and B assemblages, detected in humans (and hence potentiallyzoonotic), the other assemblages are: C and D (dogs), E (hoofed livestock), F (cats), G(rats and mice) [7].
3.1 PLASMA MEMBRANE PROTEINS
The plasma membrane is a semipermeable lipid bilayer interface important for cellsignaling and as an attachment point for the intracellular cytoskeleton. Giardia’sspecialized plasma membrane interacts with the host environment and is crucial as it isthe site of feeding (endocytosis), waste removal (exocytosis) and immune defense. Asingle layer of surface antigens coats the trophozoite and the ability to vary these coats isnecessary for survival. This cellular defense mechanism is called “antigenic variation”and it involves gene activation and silencing to produce switching among the members ofa multigene surface protein family [8], the variant surface proteins (VSP), which switchspontaneously and can be selected by immune pressure or physiologic conditions.Giardia is able to flourish in the proteolytic alkaline and bile-rich upper intestine becauseof the VSP that form a dense single molecule layer over the entire trophozoite surface,including the flagella. A striking feature of many protist variant surface antigens,including those of Giardia [9], Tetrahymena [10], and the parasitic fungus Pneumocystiscarinii [11], is the conserved periodicity of cysteine residues. Giardia VSP molecularweights vary between 20 to 200 kDa, with an estimated repertoire of between 235 to 275genes [12]. All are extremely cysteine-rich (about 12%) Type 1 integral membraneproteins with multiple CXXC motifs, and a C-terminal membrane-spanning regionfollowed by the absolutely conserved CRGKA motif. All have a characteristic extendedpolyadenylation signal, and an N-terminal signal peptide (14-17 amino acids) for ER

4
transport [13]. The majority of cysteine residues are in intrachain bridges, and thisinternal crosslinking accounts for the resistance of Giardia to harsh host digestiveconditions [14]. Only one VSP is expressed on the trophozoite surface at any one timeexcept during VSP switching, which occurs every 6.5–13 generations [15]. The initiallyexpressed VSP is gradually lost and replaced after 12–36 h [16]. Switching also occursduring differentiation (encystation–excystation) [17], offering an additional role inimmune protection. Epigenetic mechanisms are now known to be responsible for VSPgene transcription activation, instead of special DNA rearrangements [18, 19]. Very fewother membrane proteins have been identified in Giardia, but at least 278 proteins arepredicted to have a signal sequence using SignalP [20] and at least one membranespanning region using TMHMM (www.cbs.dtu.dk/services/TMHMM) (Reiner, Svard,Gillin unpublished). The heavily disulfide-bonded membrane proteins of Giardia providea dual protective role against both environmental and immune challenges, whileanchoring the parasites’ cytoskeleton.
3.2 CYTOSKELETON
Giardia’s cytoskeleton is a dynamic cellular "scaffolding" that is required formultiple processes, including nuclear division, cytokinesis, maintaining cell shape,motility, flagellar movement, and intracellular transport [21]. During mitosis, a bigchallenge is to correctly segregate the two nuclei and multiple cytoskeletalcomponents. The parasite cytoskeleton resists membrane leakage and structuralcollapse [22], and is composed of both classic cytoskeletal (microtubules andmicrofilaments) and Giardia-specific organelles and proteins [23]. There are fivetypes of microtubular structures: the mitotic spindle, flagella/axonemes, ventral disc,median body and funis. The first two are universal cytoskeletal organelles, but theventral attachment disk, median body and funis (described below) are Giardia-specific. The Giardia cytoskeletal proteins giardins [24, 25], belong to at least threeseparate gene families: the a-giardins or annexins [26, 27], b-giardin, a striated fibreassemblin (SFA) homolog [28] and g-giardin, a protein without notable homologs[29]. The presence of 21 annexin homologs in such a simple eukaryote is surprisingand emphasizes their functional importance to the parasite. Annexins in otherorganisms are calcium (Ca2+) dependent phospholipid-binding proteins, whichinteract with structural cell-membrane components, occasionally acting as atypicalCa2+ channels [30]. In Giardia, they have been localized to flagella during growth andpresumably excystation, and are close to the cyst wall during encystation, and arehighly expressed proteins [31]. b-giardin assembles into 2.5 nm filaments that furtherassemble into the superstructures of the ventral ribbons of the ventral disk [32].Extensive searches of the Giardia genome failed to identify actin-associated proteins,microfilament-specific motor protein family or myosin [12]. The main microtubuleorganizing center (MTOC) of cells, and nucleating center for flagella is the basalbody which would be expected to play a central role in the organization of Giardia’scomplex cytoskeleton and eight flagella. Giardia’s complex cytoskeleton undergoesdramatic re-arrangements during both differentiations, and it is important tounderstand, which signaling proteins localize to the cytoskeleton. Once these proteinsare identified, their specific function can be examined.

5
3.2.1 Flagella
Eukaryotic flagella are motile organelles built on a framework of nine microtubule fiberssurrounding two central microtubule fibers. The “9 + 2” axoneme, selectivelyincorporates specific receptors and ion channels, and is powered by dynein ATPasemotors [33]. Beyond simple propulsion, flagella have sensory functions asmechanotransducers and chemoreceptors. These biological “antenna” are capable of astimulus-response coupling of environmental stimuli into changes of flagellar beatfrequency mediated by a rise in intracellular Ca2+ levels ([Ca2+]i ) [34, 35]. Giardia’seight flagella are covered by the plasma membrane and arrayed in four pairs: the anterior,posterolateral, caudal and ventral, which originate from basal bodies located between thetwo nuclei [22]. Each flagellum has a characteristic motion, and exits the cell body at aspecific location. The intracellular portion of each flagellum is accompanied by a uniqueparaflagellar dense rod (PFR) while the extracellular portion is membrane-bounded [22].The kinetoplastid flagellum also contains a paraflagellar rod structure which is necessaryfor full motility and provides support for metabolic regulators that may influence flagellarbeating [36], and has homologs in the Giardia genome (Reiner, Svard, Gillinunpublished). The anterior, posterolateral, and ventral flagella beat with a similarfrequency, but with different amplitudes [37]. The caudal flagella have a single set ofdorsal and ventral short arrays of microtubules, which run parallel to the caudal flagellafrom the disc to the tip of the tail called the funis [22, 38, 39]. Giardia’s flagella areinvolved in all aspects of the parasites lifecyle. During growth they function inattachment [40], detachment, swimming, and feeding [37], They undergo a complexmigration and maturation over the course of three cell divisions (18-24 h) [41]. Duringencystation, flagella become internalized and quiescent [42]. Resumption of flagellarmotion during excystation helps enlarge the opening at one pole of the cyst, whereexcyzoites emerge, posterior end first [43]. Typically flagella are thought of as propulsionorganelles, but some could play concurrent roles in environmental monitoring and signaltransduction.
3.2.2 Basal bodies
Basal bodies are highly conserved, self-replicating, cylindrical organelles that provide thetemplate for flagellar assembly. One basal body directly nucleates each cilium(flagellum), and is thought to be involved, in cell-cycle progression, morphogenesis, andmotility [44]. Giardia’s eight flagella are each nucleated by a basal body [45], eachlocated between and slightly anterior to the two nuclei [46, 47]. Currently three signalingproteins that are required for and also upregulated in excystation have been shown tolocalize to the basal bodies: calmodulin (CaM) [48], protein kinase A (PKA) [49, 50] andprotein phosphatase 2A (PP2A) [51], with aurora kinase localizing to the basal bodiesduring mitosis [52]. Recent basal body proteomic analyses have identified many proteinsin other organisms [53-56], but until now only six other Giardia basal body proteins wereknown: a, b-, and g-tubulins, centrins 1 and 2, and ERK1 [52, 57-60]. Beyond thetraditional role as nucleating centers for flagellar biogenesis, Giardia’s basal bodies mayact as signal transduction and control centers during both growth (cell cycle) anddifferentiation (life cycle).

6
3.2.3 Ventral disk
A unique anatomical feature is Giardia’s prominent ventral attachment disk. There isa strong link between the ventral disk and disease since attachment to host epithelialcells is essential for Giardia’s ability to avoid peristaltic elimination [22, 61]. Theventrolateral flange of the disk interdigitates between microvilli of the small intestine,but is also capable of attaching to inert surfaces [62]. During growth in vitro Giardiaalternates between attached and free swimming phases, depending on the competenceof the parent or newly assembled disks [63]. Division starts in attached cells bydetachment of the disk microtubules from basal bodies, the b-giardin microribbonsare lost, and the microtubular layer unfolds, resulting in detachment. Then twodaughter discs assemble on the dorsal side of the attached cell during a free-swimming phase. Finally, the daughter cells with fully developed disks, but stillconnected tail to tail by a cytoplasmic bridge, attach to a substrate and terminate thedivision by a process resembling adhesion-dependent cytokinesis [63]. a- and b-tubulin and b-giardin, SALP-1, delta-giardin and aurora kinase all localize to theventral disk [22, 52]. b-giardin and SALP-1 (striated fiber assemblin-like protein 1)are both immunoreactive in serum from patients with giardiasis. They are also arehomologous to the striated fiber assemblins, which are microtubule-associated fibersthat emerge from Chlamydomonas reinhardtii basal bodies [64]. During encystationboth proteins localize to the four disassembled ventral disk fragments in the cyst,which is rapidly reassembled during excystation, and used by the excyzoite to attach[65]. A central theme for Giardia’s survival during growth and differentiation isdefeating peristaltic elimination.
3.2.4 Median body
The median body is a Giardia-specific cytoskeletal organelle, which has been widelyused for species classification. Proposed cellular functions of this puzzling organelleinclude: 1) a microtubule reservoir, 2) ventral disk progenesis, 3) immobilization ofmicrotubules between cell divisions, 4) a microtubule nucleation site and 5) vertical“tail” flexing [66]. By light microscopy, the median body in the trophozoite is shapedlike a claw hammer [67], in electron micrographs it is seen as roughly aligned“fascicles” (bundle or cluster) [22] and like clusters of unorganized microtubules inthe cyst [68]. Because the presence of median bodies varies between 50-86% ininterphasic populations, a role in Giardia’s cell cycle has also been proposed [66, 69].The Ca2+-binding basal body protein centrin and the ventral disk protein b-giardin,aurora kinase and an uncharacterized coiled-coil 101 kDa median body-specificprotein [70], have been localized to this non-membrane bounded organelle [52, 58, 59,71]. Post-translational modifications of median body-specific tubulin includeacetylation, mono and polyglycylation, and tyrosinylation [72-74] have been observed.Whatever its eventual function proves to be, it is quite curious that the median bodyshares proteins with both the basal body and ventral disk.

7
4 DIFFERENTIATION
Differentiation in Giardia involves two very different developmental transitions, from themotile, replicative trophozoite to the dormant cyst (encystation), and from the ingestedcyst to the emergent excyzoite (excystation), which is accomplished by exiting from andre-entering the parasite’s cell cycle [75-77]. During differentiation there is a regulatedswitching of gene expression programs in response to host and environmental stimuli.Giardia’s differentiations are among the simplest eukaryotic developmental processesknown and are experimentally tractable [78-81]. Equally important, Giardia’s completedin vitro lifecycle is and excellent system for modeling other uncompleted parasitelifecycles and for studies of protozoan differentiation in general.
4.1 ENCYSTATION
The cyst is essential for survival of the parasite outside the host [82] and the persistenceof endemic infection is due to the transmission of the cyst from host to host through fecalcontamination. Encystation is the gradual transformation of the motile trophozoite intothe immotile, dormant and refractile cyst in response to host-specific factors [42]. As thetrophozoites’ flagella are internalized, the parasite loses the ability to attach [83], theventral disk fragments [65] and the differentiating encyzoite gradually rounds up andenters into a hypometabolic dormancy. The cyst wall is the environmentally resistant, butactively transducing, biological boundary responsible for Giardia’s survival duringdormancy. The highly insoluble nature of the Giardia cyst wall (CW) is due to the strongcarbohydrate interchain interactions and cyst wall sugar associations with cyst wallproteins. The wall is synthesized de novo from endogenous glucose via a pathway ofinducible enzymes, which are transcriptionally as well as allosterically regulated [82].The synthesis of cyst wall proteins (CWP) that begins early in encystation leads to theformation of novel large encystation secretory vesicles (ESV) [84-87], which are theCWP export organelles. Late in encystation, after the cyst wall has been laid down,nuclear division occurs producing the four cyst nuclei [84, 88]. Giardial encystation isreminiscent of the sexual process of meiosis [75]. Until recently, Giardia has beenconsidered strictly asexual, but the presence of meiotic genes [12, 89], low levels ofallelic heterozygosity [90], and new evidence of recombination from population genetics[91] argues otherwise. Logsdon recently reported that to “unlock sex” in Giardia, weneed investigations of genetic exchange at the cellular level [92, 93] and to “catch themin the act” [94]. Recent evidence for this suggests that encystation-specific karyogamy(fusion of nuclei), with subsequent somatic homologous recombination occurs in cysts.This ancestral parasexual process, is coined “diplomixis”, and is presently unique toGiardia, but predicted to occur in other diplomonads [95]. Both pathogenic microbes andparasites are beginning to disclose some shared principles. By limiting genetic exchange,but retaining the sexual or parasexual reproduction machinery, pathogens are able togenerate highly clonal, but at the same time, recombining populations. This is aconstantly changing balance between clonality versus diversity, and is in direct responseto host, environmental, or antimicrobial challenges [96]. Encystation therefore is a keyvirulence and survival mechanism with a fine-tuned genetic response to host stimuli.

8
4.1.1 Encystation stimuli
It was long observed that there is a relationship between bile secretion and the numberof colonizing trophozoites [97], and when a low concentration of bile is added toparasite culture medium, trophozoite growth increases dramatically [98, 99]. Wehypothesized that mimicing the human upper intestinal environment would triggerencystation, and we were the first to observe encystation in vitro after adding highconcentrations of bile at the physiologic pH 7.8 [78, 80]. Cyst formation hasalternatively, been induced in vitro by starving trophozoites of cholesterol. The physicalstate of the bile salt molecules in solution (monomers or micelles) was suggested as thestimulus of encystation [100]. When physiological levels of bile are reduced in micewith giardiasis, using either surgical cholestasis or feeding a diet of bile-binding resins(cholestyramine), significant reductions (103-104) in fecal excretion of Giardia cysts areobserved [101]. Whether encystation is induced by high amounts of bile directly or bycholesterol deprivation, or whether “It is likely that the former induces the latter” [102],there is still no identified molecular mechanism for induction of Giardia encystation.
4.1.2 Cyst wall biogenesis
Giardia’s cyst wall is the single structure that protects and preserves the parasite duringdormancy. It is a model immunoprotective extracellular matrix excluding smallmolecules like water while efficiently transmitting host stimuli. The fibrillarextracellular matrix is lined by a double inner membrane and composed of an outer 300nm thick filamentous wall [103], which is 43% carbohydrates [104], at least 86% ofwhich is a b(1–3)-N-acetyl-D-galactosamine homopolymer [82]. The cyst wall filamentsmeasure 7–20 nm in diameter and are arranged in a tightly packed meshwork [105]. Thewall is assembled during encystation by the encyzoite using an elaborate CWP secretorysystem [85, 86, 106, 107]. There are currently four known CWP’s, three of which areleucine-rich repeat-containing proteins with positionally conserved cysteine residues,while the fourth resembles trophozoite variant surface proteins (VSP) [108-111]. Allform extensive intermolecular disulfide bonded complexes, are sorted, concentrated andexported to the nascent CW by encystation-specific vesicle (ESV), but theirsupramolecular architecture is incompletely understood. CWP are either synthesized ina dilated ER cisternae region known as the cleft [87, 88, 106], which gradually widens toform the novel (ESV), or ER vesicles containing CWP use each other to form the ESV[85, 112]. The importance of the ESV cargo is supported by our finding that chemicallyreducing these complexes in situ with DTT reversibly disrupts the ESV [113],transforming them to flattened ER-like cisternae [114]. The ESV contents must attaintheir insoluble architecture after secretion [115]. Several enzymes, mainly localized tothe ESV are needed for post-translational modifications of the CWPs that have beenimplicated in CW formation. These include three protein disulfide isomerases [116], alysosomal cysteine proteinase [117] and a Ca2+-binding granule-specific protein(gGSP). Knockdown of gGSP inhibits ESV release, suggesting a Ca2+-dependentprocess [118]. Thus, a number of independent studies show that the ESV are central toCW biogenesis, since any manipulation that interferes with the ESV pathway, blocks alldownstream events. Despite cellular differences between the human intestinal parasitesEntameba histolytica and Giardia lamblia, and the opportunistic free-living soilamoeba Acanthamoeba castellani, recent morphological studies support the idea that

9
ESV’s may correspond to a common mechanism of synthesis, transport, and depositionof cyst wall components [119].
4.2 CYST
Giardia lamblia cysts are nonmotile and oval shaped with refractile walls. They measure8-12 mm long by 7-10 mm wide and are secreted in the feces. The first ultrastructuralobservation of Giardia cysts revealed a thin layer of cytoplasm separating the wall fromthe cyst periphery, with four nuclei and basal bodies in between and ribbon-likemicrotubule structures from disassembled attachment disks and flagella [120].Environmental communication is essential for cyst survival and even after being dormantfor months in water, they are poised for rapid awakening upon ingestion. Proteinsrequired for excystation are upregulated during encystation, and stored within the cystbody. The parasite emerges in or near the duodenal region after passage through thestomach in a regulated awakening from dormancy. The cysts’ re-entry into the cell cyclerequires accurate transduction of host signals and timing of cellular re-assembly. Thisdifferentiation, called excystation is a crucial, but incompletely understood, aspect of thegiardal lifecycle.
4.3 EXCYSTATION
Excystation is a dramatic awakening from dormancy that is required to initiate infection.excystation in vitro involves at least a two-step process that is initiated by exposing cyststo “conditions closely approximating the organism's in vivo environment", an acidicenvironment, [121, 122] followed by the slightly alkaline and proteolytic conditionsmimicking the duodenal-jejunal region [81, 123, 124]. Excystation is a rapid process[125], the cyst is first triggered to excyst by host stomach acids or activation (5-10minutes), and then passes into the small intestine before rupturing (20-60 seconds).During the rapid emergence (<1 minute) from the cyst, flagella first appear through anopening in one of the poles of the cyst, followed by the excyzoite body [75].Ultrastructurally, one can see novel protrusions of clear cytoplasm near the flagella calledthe preventral flange which are thought to help in establishing the polarity of theexcysting cell [68]. Cytokinesis is centrally important to excystation, the pleomorphicexcyzoite goes through one round of cytokinesis to give two daughter cells (7-8 min),which divide again, finally producing four diploid trophozoites [75]. The excyzoite mustsimultaneously divide, segregate its organelles, activate its motility and attachmentapparatus while increasing its metabolism [51]. Biochemically during excystation, CWPare dephosphorylated [126] while endogenous cysteine proteases are released into theperitrophic space [124], and excystation-specific transcripts are expressed [68]. Many ofthe expressed transcripts are VSP genes involved in the switching of surface coats duringdifferentiation [17]. Giardia has at least three sets of genes whose expression is uniquelyregulated. encystation-specific genes that are expressed only as trophozoites differentiateinto cysts, excystation-specific genes that are expressed by the excyzoite duringexcystation and VSP that are expressed one at a time on the surface of the trophozoite.The accurate transduction of environmental signals and timing of cytoskeletal re-assembly depends on a sophisticated signaling system.

10
5 CELL CYCLE
The cell cycle is the series of events that take place in a eukaryotic cell leading to itsreplication. These events can be divided into two brief periods: interphase—during whichthe cell grows, accumulating nutrients needed for mitosis and duplicating its DNA—andthe mitotic (M) phase, during which the cell splits into two distinct "daughter cells". Thefundamental task of the cell cycle is to make sure that the genome is replicated once andonly once in every cell cycle (S-phase) and that the chromosomes are distributed equallyto the daughter cells (M-phase). In between these two phases is the gap phase 1 (G1),when preparatory events for DNA replication are initiated and gap phase 2, (G2), whenproteins needed for mitosis are accumulated. Giardia is unusual in that it contains twoapparently identical, synchronously replicating nuclei in the vegetative trophozoite stage[127, 128]. Giardia’s cell cycle and especially the timing of DNA replication andcytokinesis are central to its differentiation [76]. The length of Giardia’s cell cycle invivo is unknown, but factors that would shorten this time can be considered virulencefactors. Until recently, the investigation of Giardia’s cell cycle has been hampered by thelack of a cell synchronization method [127, 129-131]. Most cell synchronization methodsuse drugs that reversibly arrest and release cells in the same phase, allowing accurateanalyses of cellular events and gene expression during the cell cycle. There are tworecent reports of successful synchronization of Giardia [77, 132], one of which identifiesfor the first time (Paper 3) Giardia’s differentiation restriction point, or the place in thecell cycle where Giardia preferentially arrests from growth to encyst.
5.1 GROWTH
Host or parasite factors that increase the growth rate of Giardia during an infection canbe considered virulence factors, but those factors are not understood. Our currentunderstanding originates from cultivation and in vitro studies of Giardia growth. Thecultivation of intestinal protists in vitro has a long history without which few basicstudies could be performed beyond simple morphological or pathological descriptions.Giardia was first cultivated by Karapetyan in 1960 in a mixed culture with Candidaguilliermondi and chick fibroblasts, and Meyer was the first to report axenic cultivation(without other metabolizing cells) of human Giardia [102]. The pioneering protozoanculture work of Diamond is responsible for developing the extremely versatile TYI-S-33medium, used for the axenic cultivation of Giardia. Many basic parameters (reducingagents, oxygen, serum, temperature and such) of Giardia growth and attachment werefirst studied by Gillin’s group, who also developed an agar cloning method used for thetesting of antimicrobial compounds, both chemical and natural [133-139]. With theseimportant tools in place, deeper understanding of Giardia’s cell cycle and eventuallydifferentiation in vitro was made possible.

11
5.2 PLOIDY
Ploidy is a measure of the number of sets of chromosomes (haploid =1 N) or genomeequivalents in a cell. Giardia’s cell cycle and lifecycle are examples of a regulatedgenetic program of cycling polyploidy. During vegetative growth, each nucleus cyclesbetween a diploid (2N) and tetraploid (4N) state, resulting in cellular ploidy of 4N and8N, and during stationary phase trophozoites arrest in the G2 phase with a ploidy of 8N[76]. To encyst, a G1 trophozoite goes through two successive rounds of chromosomereplication without an intervening cell division event [76]. Late in encystation the twotetraploid nuclei divide [88] and the DNA is replicated generating a quadrinucleate cystwith a ploidy of 16N (Table 1) [75, 76]. This ‘alternative’ cell cycle, or endoreplication(endoreduplication) occurs after Giardia completes two successive rounds of DNAreplication without an intervening mitosis. It is a special cell cycle (endocycle) ofterminal differentiation, and is often seen in the genomes of specialized biosyntheticallyactive cells [140, 141]. Endoreplication permits growth without periodic rearrangementsof cytoskeletal elements, since chromosomes are not required to segregate and aretherefore extremely resistant to DNA damaging conditions [142]. Fully differentiatedcysts contain four tetraploid nuclei (16N), as does the excyzoite. Upon emergence fromthe cyst, the excyzoite divides twice, eventually forming four trophozoites with twodiploid nuclei each [76]. Giardia’s unique cellular status, (binucleate and with cyclingploidy) during growth, and special cell cycles, renders many classical genetic andmolecular approaches useless. Therefore special bioinformatic and computationalapproaches are necessary for deeper understanding of this unique important parasite.
Table 1. Summary of host stimuli and Giardia’s known cellular responses during its lifecycle.The cellular ploidy cycles between 4N (G1) and 8N (G2) during growth * The average pH of unfilteredstream/river water in the United States in 2007 from 39,725 samplings from 25,039 sites (avg. 7.78 +/- 0.6)(DSR unpublished; http://water.usgs.gov/)

12
6 COMPUTATIONAL BIOLOGY
Because Giardia is polyploid, classical genetic methods are not well-suited. Therefore,genomic, transcriptome, and proteomic analyses become especially valuable.Bioinformatics and computational biology are interdisciplinary approaches that usemathematical tools to extract useful information from data produced by high-throughputbiological techniques. Bioinformatics concentrates on the management and analysis ofbiological data, while computational biology is mainly concerned with hypothesis-driveninvestigation of a specific biological problem (http://www.bisti.nih.gov/). Many parasitegenomes have been sequenced, most derived from protozoa [143]. The parasite genomesequence, by itself, cannot provide a full explanation of organismal biology. It is a firststep in the gleaning of basic cell-biological information, and needs to be combined withother high-throughput technologies such as transcriptomic, proteomic, protein-protein/DNA interactions. Ideally these powerful tools need to be applied at various timesin the life cycle or in response to specific stimuli. The resulting high-dimensional datasets can then be used for large-scale interaction mapping and cell biological validation.Major efforts using network models are ongoing to understand the gene products andtheir interactions in the growth, development and survival of parasites [144]. The resultsfrom these technology intensive studies will provide a deeper insight into thepathogenesis and progression of parasitic diseases [145, 146]. The tools of biologicalresearch are rapidly expanding. Not only are we in possession of the blueprints for onetype of information (genome), but we are also beginning to understand how this encodedinformation helps cells transduce the dynamic information in their environments [147].
6.1 GENOME
The genome of an organism is a complete DNA sequence of one set of chromosomes.The genome size in eukaryotes varies 200,000-fold with symbionts, intracellularpathogens and some obligate parasites being among the most compact known [148]. Thesequencing of the genome of the parasitic diplomonad Giardia lamblia WB (assemblageA) is now complete [12] and reveals ~12 million base pairs distributed on fivechromosomes. There are currently 6,500 predicted open reading frames with 73% shownto be transcribed by SAGE studies. The total number of contigs is currently 306, with anaverage shotgun coverage of approximately 11-fold equaling 96% closure. Thesequencing of the genome and the initial website hosting was done at the MarineBiological Laboratory, Woods Hole and the current genome assembly GiardiaDB(http://www.giardiadb.org ) was released in April 2007, and is part of the EukaryoticPathogens Database Resources (http://eupathdb.org/eupathdb/). The major conclusionfrom analyses of the giardial genome is that it is compact in structure and content. Itcontains only 4 known introns, a few mitochondrial relics and a simplified archaeal-likeDNA replication machinery. It has a yeast-like machinery for transcription and RNAprocessing and a limited set of largely bacterial-like metabolic enzymes [12, 149].Excluding the NEK (NIMA-related family of serine/threonine kinases), Giardia’s kinomeis the most compact known from any eukaryote. Giardia trophozoites have ampleopportunity to pick up genes from bacteria and to scavenge products of host and bacterialmetabolism and the genome contains many lateral gene transfer (LGT) candidates (95; ≤E value -30). Because Giardia is a binucleate tetraploid protozoan, a high level of

13
genome heterozygosity could accumulate, but surprisingly this is estimated to be lessthan 0.01%. The recent report of Poxleitner et al [95] may help in identifying thebiological mechanism used for maintaining genome fidelity between the four genomecopies [12]. The minimal nature of Giardia’s genome will help in dissecting the complexmultiprotein pathways and cellular processes in other eukaryotic cells by helping todefine simple model systems
6.2 TRANSCRIPTOME
Giardia is one of a handful of parasites whose complete life cycle can be reproduced invitro. This provides an excellent functional model for parasites lifecycles that have notyet been replicated. The transcriptome is the set of all messenger RNA (mRNA)molecules, or "transcripts", produced in one or a population of cells. Giardialtranscriptome analyses are especially important because of the insight they provide inunderstanding parasite responses to life cycle events and changing environmental stimuli.Expression of many giardial genes appears regulated at the transcriptional level. Becausegiardial mRNAs have several unusual features, its transcriptional regulation is of especialinterest. Giardia mRNAs’untranslated regions are unusually short (generally 0-14nucleotides) and most Giardia mRNA molecules do not appear to be capped. There islittle similarity of Giardia promoter regions with known eukaryotic regulatory elements[150] and approximately 20% of the total polyadenylated RNA is believed not to containORFs or encode protein [151]. Because many of the intergenic regions are less than 100base pairs in length, the very compact nature of the genome might prevent the general useof longer 5'-untranslated regions [152].
One exception to this is the glucosamine-6-phosphate isomerase B (Gln6PI-B) gene thathas two transcripts, one is expressed constitutively and the second is highly upregulatedduring encystation. The non-regulated Gln6PI-B transcript has the longest 5'-UTR knownfor Giardia and is 5' capped or blocked. In contrast, the Gln6PI-B upregulated transcripthas a short, non-capped 5'-UTR. A small promoter region (< 56 bp upstream from thestart codon) is sufficient for regulated expression. Gln6PI-B also has an antisenseoverlapping ORF that is constitutively expressed and a shorter antisense transcript that isdetected during encystation. This is the first report of a developmentally regulatedpromoter in Giardia, as well as evidence for a potential role of 5' RNA processing andantisense RNA in differential gene regulation [153]. gMyb2 is the first giardialtranscription factor to be functionally identified and the first to be associated withupregulation of encystation genes. This work provides a model for study of differentialgene regulation in early diverging eukaryotic organisms. [150]. Excystation is a time ofcomplex changes in mRNA species and specific transcripts appear, disappear, or changein abundance at each stage of the lifecycle as first shown by differential mRNA displayanalysis [68]. Unlike the genome, the transcriptome varies with external environmentalconditions and reflects the genes that are being actively expressed at any given time. Ahigh-throughput transcriptomics project using serial analysis of gene expression (SAGE)was used to explore Giardia transcription at 10 different points throughout differentiation[65]. SAGE (Velculescu, Zhang et al. 1995) is a technique used by molecular biologiststo produce a snapshot of the messenger RNA population in a sample of interest, andprovides quantitative information on gene expression. Unlike microarrays, SAGE iscapable of detecting unknown transcripts, since it does not require a priori knowledge of

14
what is present in the sample being analyzed. Presently only the trophozoite stage ispublicly available at NCBI (GEO accession number GSE8336). We have collaborated inthe SAGE project and already the data on transcript levels over the life cycle have beenextremely useful in understanding cytoskeletal dis-assembly [65], identifying adifferentiation-specific cyst protein [111], and phosphatase 2A subunit C [51]. TheSAGE data has also been used in this study in analyzing Giardia’s basal body proteomeduring differentiation (Paper IV) and discovery of a high cysteine non-variant cystprotein (HCNCp) (Paper II). Future challenges will involve deciphering the regulationand functions of differentiation-specific genes.
6.3 PROTEOMICS
The ultimate goal of proteomics is to enhance genomic and transcriptomic efforts tounravel biological processes and functions. Although gene transcription is very valuableit gives only a rough estimate of protein expression levels due to mRNA degradation,translation inefficiencies, alternative splicing and post-translational modifications.Finally, many proteins form complexes with other proteins or RNA molecules, and onlyfunction in their presence. A surprising finding of the Human Genome Project is thatthere are far fewer protein-coding genes in the human genome than proteins in the humanproteome approximately 25,000 genes versus 500,000 to 2 million proteins(http://www.hprd.org/), which dramatically illustrates the importance of proteomics.Proteomics relies on: genomics, cell fractionation, protein separation sciences, massspectrometry and bioinformatics [154]. Subcellular proteomics involves the use ofmethods, such as sucrose density gradient fractionation and immuno-affinity purificationof organelles or complexes prior to any proteomics approach. This is a powerfuladditional tool, as it reduces the sample complexity, while maintaining the structural orfunctional properties of the complex under study [155]. For Giardia, subcellularproteomics is in its infancy with only two published studies and the third (Paper 4)analyzing basal bodies. The differentiation-specific organelle, the ESV was studied byfirst isolating the organelles using sucrose density gradient fractionation and separatingusing two-dimensional gel electrophoresis (2-DE) which separates protein mixtures in thefirst-dimension using the protein’s isolectric point, then in the second-dimension usingmolecular weight. 2-DE is a powerful tool both as a sensitive separation method, but alsoallows identification of functionally important peptides (spots) by probing blots withantibodies or other ligands. After separation and staining, matrix-assisted laserdesorption/ionization (MALDI) [156] was used for identification of ESV peptides.Sixteen different proteins were identified as being enriched in ESV’s, and a model ofESV genesis and maturation was proposed [114]. In the second study, immunodominantantigens during acute giardiasis were identified using a co-culture model, to test thehypothesis that contact of Giardia with intestinal epithelial cells might lead to release ofspecific proteins. After controlled cell-cell interactions, proteins were precipitated fromculture supernatants and analyzed as above, using 2-DE and MALDI [157]. For theproteomic analysis of Giardia basal bodies and CaM-binding proteins in this study (PaperIV) a highly sensitive, high-throughput technique Multidimensional Protein IdentificationTechnology (MudPIT) was used for peptide identification. MudPIT was developed byYates and colleague, and couples biphasic or triphasic microcapillary columns to high-performance liquid chromatography, tandem mass spectrometry, along with automateddatabase searching [158-161]. Using subcellular separation followed by mass

15
spectrometry we identified unambiguously 310 candidate Giardia basal body proteins.For the analysis and interpretation of this complex proteomic dataset, mathematical andbioinformatic analyses are essential [154], and elegantly combined using biologicalnetwork analysis.
6.4 INTEROLOG NETWORK
Discrete biological functions are rarely attributed to an individual molecule. Instead, mostbiological characteristics arise from complex interactions between the cell's numerousconstituents. At an abstract level, network components can be reduced to a series ofnodes connected to each other by links (edges), with each link representing theinteractions between two components. In our model the nodes are basal body-specificGiardia proteins and the putative links are inferred. This is accomplished by usingorthologs of other model organisms that have experimentally determined protein-proteininteractions or an “interactome”. Orthologs are genes in different species that aresimilar to each other and often, but not always, have the same function. An interolognetwork then is built by combining curated interactions from the experimental organismwith known interactions transferred from a model organism [162]. To use this approach,we first identified Giardia orthologs in humans using reciprocal BLAST (Basic LocalAlignment Search Tool) best-match searches and phylogenomic analysis. The use ofhuman datasets is especially valuable because of the well-established human protein-protein interactome. The ortholog pairs from known human interactions are then used foridentifying potentially conserved interactions in Giardia. The interolog network methodprovides a rich source of annotated and experimentally verified interactions for furtherhypothesis generation and model refining. Importantly, this opens the possibility ofidentifying and understanding highly conserved eukaryotic processes, between Giardiaand humans. The power of this approach was confirmed in a study of the minimalproportion of true interologs between Saccharomyces cerevisiae and Caenorhabditiselegans. Although the minimal proportion of true interologs varied between 16% and31%, this was still about 103 times higher frequency of interactions than obtained usingconventional two-hybrid screens and random libraries [163]. Literature mining can thenbe used for validating the predicted network, which in the case of C. elegans confirmed~7% of the predicted interologs from signal transduction pathways or molecularmachines [164]. Since cellular networks are governed by universal laws they offer a newconceptual framework [165], for the study of parasite biology.

16
7 AIMS OF THE PRESENT STUDY
I Determine the role of calcium signaling during differentiation.
II Identify new differentiation-specific proteins.
III How can Giardia be synchronized?Can we identify a differentiation restriction point?
IV Identify the differentiation regulation center.

17
7.1 PAPER I
Cysts lay dormant for long periods of time, but after ingestion by the host, are able totransduce host signals and differentiate. Higher eukaryotic cells use intracellularsignaling, which requires messengers whose concentration varies with time. In thisproject, we proposed the broad hypothesis that Ca2+ is the premier signaling molecule forused for signal transduction [166] and control of the cell cycle. Ca2+ signals aretransduced by CaM, and Ca2+/CaM-dependent pathways and influence several importanttargets of the cell cycle [167]. Paper I tests the specific hypothesis that examines the roleof Ca2+ signaling during differentiation.
For eukaryotic cells, Ca2+ signal transduction and the Ca2+ sensor calmodulin are crucialfor regulation of all aspects of cellular physiology during both growth and differentiation.The giardial Ca2+ regulated activities include flagellar motility, enzyme activity, andcoordination of its complex cytoskeleton. The highly local nature of Ca2+ signaling ismediated by specific cellular proteins that bind Ca2+ with over a million-fold range ofaffinities (nM to mM), enabling cells to using this binding energy for signal transduction[166]. We hypothesized that Ca2+ homeostasis would be vitally important in the rapid re-assembly of Giardia’s complex cytoskeleton during excystation. In support of this, weshowed that both the extracellular (EGTA, 5 mM) and [Ca2+]i chelators (BAPTA, 5mM)strongly inhibited (>70%) excystation, implicating Ca2+ homeostasis as an importantfactor for excystation. We hypothesized that the greatest effects of Ca2+ perturbationswould be during cellular activation that occurs late in excystation during emergence,when the excyzoite becomes motile and goes through rapid cytokinesis. To test thathypothesis, we used specific inhibitors of the cellular machinery required for maintainingCa2+ homeostasis. We first lowered [Ca2+]i
levels using the Ca2+channel blockerverapamil , and found strong inhibition (>75%) of excystation, specifically in Stage II.We then raised [Ca2+]i
levels by allowing Ca2+ influx from the culture medium, using twodifferent Ca2+ carboxylic ionophores, ionomycin (1 mM) and A23187 (1 mM), we againfound the greatest inhibition during Stage II. This confirmed the importance of Ca2+
signaling and homeostasis during differentiation, specifically when the excyzoite isemerging from the cyst during excystation.
Ca2+ easily precipitates phosphate, and cells invest much energy to exert control overCa2+, by either chelating, compartmentalizing, or extruding it, to maintain a 20,000-foldgradient between their intracellular (~100 nM free Ca2+) and extracellular (mM)concentrations. These gradients are maintained by Ca2+ pumps which are Ca2+ ATPaseswhich transfer Ca2+ from the cytosol of the cell to the lumen of the endoplasmicreticulum at the expense of ATP hydrolysis [166]. Cellular control of cytosolic Ca2+ isregulated in other cells by the endoplasmic reticulum (ER) Ca2+ pump called SERCA(Sarco Endoplasmic Reticulum Ca2+ATPase) which plays a vital role in cell growth anddifferentiation [168]. We hypothesized that Giardia had a SERCA pump which playedthe same vital role during its growth and differentiation. SERCA pumps are specificallyinhibited by the selective and irreversible sesquiterpene lactone thapsigargin (TG) [169].The lipophilic TG crosses cell membranes and prevents sequestration of Ca2+ from thecytosol, causing a massive secondary influx of extracellular Ca2+ into the cell [170].Therefore TG is a highly versatile tool for studies of [Ca2+]i
levels including inhibitionof [Ca2+]i dependent functions and localization of cellular Ca2+ stores. To look at the

18
effects of by the SERCA pump we used Indo 1, a fluorescent Ca2+ dual-emission dye[171]. As Ca2+ progressively increases from 100 to 1000 nM, the peak emission of Indo 1(a UV-excitable fluorescent Ca2+ sensor) decreases, and shifts to lower wavelengths. It isthen possible to derive the [Ca2+]i by measuring the intensity of emission at twowavelengths (405 and 480 nm), and calculating the ratio of intensities at thesewavelengths [172]. We added TG (5 mM) to trophozoites, cysts, or excysting cells thatwere pre-loaded with Indo-1. This immediately produced a sustained rise in fluorescence,corresponding to an increase in free [Ca2+]i . We found that Giardia maintains a low basalcytosolic Ca2+ concentration, ranging from 40 to 85 nM, and that the addition of TGcaused significant increases in cytosolic Ca2+ levels. The magnitude of peak Ca2+ TG-induced release increased during excystation and TG (10mM) also inhibited excystationstrongly at Stage II. Fluorescently labeled TG was used to identify a likely Ca2+ storagecompartment in cysts. This confirms that Ca2+ is stored in the cyst and that [Ca2+]i levelsin Giardia are maintained by SERCA pumps throughout growth and differentiation andagain emphasizes the importance of Ca2+ signaling during emergence, late in excystation.
The professional Ca2+ chelator in proteins is the EF hand domain (named after the E andF regions of parvalbumin). The affinities of EF hand domains for Ca2+ vary ~100,000-fold [173]. Ca2+ binding regulates protein function by triggering shape changes in EFhand domains, and altering local electrostatic fields, efficiently amplifying a molecularsignal to the size scale of proteins [174]. Calmodulin is the archetypal Ca2+ sensor andCa2+ adaptor protein [166] and one of the most highly conserved proteins in nature. It waspreviously known that CaM and PKA were involved in excystation [49, 175], thatendogenous CaM is present at a level sufficient to activate cyclic AMP phosphodiesterase[176]. We hypothesized that Giardia’s CaM would play an important role duringexcystation especially during emergence, because of our previous experiments. We foundthat three different specific calmodulin inhibitors were indeed effective at blockingexcystation, especially during excyzoite emergence. The calculated IC50 values were:chlorpromazine (28 mM), trifluoperazine (15 mM) and calmidazolium (1 mM). Weidentified Giardia’s CaM homolog (gCaM; GL50803_5333, 2.4e-52) using the genomedatabase and cloned it. When gCaM was expressed with either an N or C-terminal AU-1epitope tag it specifically localized to the basal bodies in trophozoites and cysts. This isan important finding, because it suggests that the basal bodies are a Ca2+/CaM-regulatedcellular control center in Giardia, with a special role in awakening from dormancy(excystation) to re-enter the cell cycle.
7.2 PAPER II
Currently there are only three known giardial cyst wall proteins, one of which CWP3(GL50803_2421) we previously identified using database mining [110]. We hypothesizedthat more than three CWP would be required to provide the necessary sophistication andaccurate signal transduction required during Giardia’s differentiation. We used abioinformatic approach to identify a novel cyst protein and found that it was part of alarger group of unique cysteine-rich proteins. Paper II describes the bioinformatic,molecular and cellular approaches that were taken to both validate and classify thispotential differentiation-specific gene family.

19
We first mined the SAGE transcriptome database for expression patterns suggestive ofencystation or cyst wall specific genes, and identified one (GL50803_40376) for furtherfunctional validation. The encystation-specific SAGE expression profile was confirmedusing Northern analysis, and we named the 169, 320 kDa (1609 aa) gene product,HCNCp. HCNCp strongly resembles the 235-275 genes encoding classical giardial VSPprotein family: cysteine-rich (13.9%), Type 1 integral membrane protein, multiple CxxCand GGCY motifs, a typical N-terminal cleavage site, a C-terminal membrane spanningregion and a short cytoplasmic tail. Distinct from VSP’s, however, HCNCp has adivergent C-terminal transmembrane (TM) region, and an unusual C-terminal amino acidtail, CRRSKAV versus the CRGKA motif found in every VSP. To test the hypothesisthat HCNCp would be increasingly expressed on the trophozoite’s membrane duringencystation, HCNCp was expressed under its own promoter with a C-terminal AU1epitope tag for monitoring expression and localization during encystation. Inimmunoblots similar to the stable mRNA levels, HCNCp (170 kDa) was faintly detectedin trophozoites, increasing at 21-42 hours with the highest levels in cysts. There were alsoapparently physiologically processed C-terminal fragments of 21 and 42 kDa with thesame pattern of protein expression. In vegetative trophozoites, HCNCp localizes to thenuclear envelopes/ER. During encystation, HCNCP traffics through ESV’s, but unlikethe exclusive cyst wall localization of CWP1-3, HCNCp localizes to the excyzoite cellbody. This not only identifies a new cyst protein but also suggests that the cysteine-richprotein families in Giardia may be necessary for environmental survival both duringgrowth and differentiation. If confirmed, this would also be the first excyzoite-specificprotein and an excellent vaccine candidate due to its invariant nature.
To determine whether HCNCP is a variant protein, we used HCNCp-specific internalPCR primers and amplified genes and mRNA transcripts from 7 different Giardiaisolates from Assemblage A including WB C6 subclones, unrelated human isolates, onedog isolate and one isolate from Assemblage B, and detected HCNCp expression in allexcept the Assemblage B isolate (GS/M). Assemblage A isolates are highly homogenous,while assemblage B isolates are genetically highly variable [6]. This suggests thatHCNCp is not a classical VSP, but a new invariant cyst protein.
Using custom Perl scripts (McArthur, unpublished) all proteins ≥ 10% cysteine wereidentified and this high cysteine membrane protein (HCMp) dataset queried with regularexpressions modeled after HCNCp; ≥400 amino acids, and ≥ 20 CxxC or CxC motifsminus the CRGKA motif. CXC motifs are rare in VSP’s, but repeated 8 times in HCNCp.TMHMM (http://www.cbs.dtu.dk/services/TMHMM/) [177] was used to identify onlythose HCNCp-like proteins with a single transmembrane domain. We found an additional60 large HCMp’s that fulfilled these criteria. HCMp’s are rarely found in other parasitesor model organisms with the exception of the intestinal parasite Entamoeba histolytica (6HCMP’s) and the free-living ciliated protozoan Tetrahymena thermophila (30 HCMp’s),which is capable of switching from commensal to pathogenic modes of survival. If thegenome size is considered, based on predicted ORFs, T. thermophila has 5 times fewerHCMp than Giardia. Interestingly, after Paper II was published, a genome survey of theclosely related diplomonad Spironucleus salmonicida, which infects cultivated salmon,was reported [178]. The predicted amino acid sequences of 149 Spironucleus genescontaining more than 10% cysteine, with multiple CXXC, but no CRGKA and GGCYmotifs. Less than 10% of the cysteine-rich proteins in Spironucleus showed highest

20
sequence similarities to Giardia, but were most similar to proteins of the ciliate T.thermophila. This suggests that cysteine-rich proteins in diplomonads vary greatly andthat the gene families appear to have expanded independently in the Giardia andSpironucleus lineages [178]. Although most members of the genus are parasites of fish,birds and mice, essentially nothing is known about their virulence mechanisms [178].Spironucleus vortens has been cultivated in vitro and grows in Giardia culture medium ata remarkably wide range of temperatures (5 –340 C). Cysts isolated from fecal samples ofSpironucleus muris have been excysted, but the in vitro lifecyle has not been completedin any Spironucleus genus. The finding that HCMp/HCNCp-like proteins exist in a widerrange of diplomonad protozoans, strengthens Giardia’s unique position as modeldifferentiation organism and more importantly, for understanding how to completelifecycles in other diplomonad protozoans of economic and medical importance.
The ability to infer novel motifs that define a putative protein family is not trivial andrequires the combination of multiple alignment methods with human pattern-recognitionskills [179]. Biological motifs are more than just conserved nucleotide or amino acidstrings, but have underlying structural properties among the seemingly diverse consensussequences. We hypothesized that the 60 HCMp’s identified using the sequence structural“rules” of the cyst wall protein HCNCp (i.e. ≥400 amino acids, ≥ 20 CXXC or CXCmotifs minus the CRGKA motif), possess either functionally or spatially equivalentfeatures that can be detected. We first used the open-source probabilistic modelingsoftware HMMER [180]. An HMM (Hidden Markov model) transforms the informationcontained within multiple sequence alignments into a position-specific scoring system,that is used for sensitive database searching. HMMER (http://hmmer.janelia.org/)software was used to build custom HMM’s, or to use professionally curated HMM’s(Pfam; http://pfam.janelia.org/ [181]). Custom and curated HMM’s were used to searchfor common themes or subgroups within the giardial HCMp dataset. We found 6 E.histolytica HCMp’s annotated as transmembrane kinases (TMK). The TMK’s have beenpostulated to be involved in sensing and responding to extracellular stimuli [182] in E.histolytica. We used the non-kinase extracellular domains to build a custom TMK HMM.We used PFAM’ search for HCNCPs resembling VSP (PF03302) and epidermal growthfactor (EGF-like) (PF00008) from the PFAM database. A common feature of the EGF-like repeats (39-40 aa) used to build PF0008 is that they are found in the extracellulardomain of membrane-bound proteins or in secreted proteins. Together these findingssuggest that the HCMp’s have extracellular domains, which are possibly involved intransducing environmental stimuli. MEME (Multiple Em for Motif Elicitation)/MAST(Motif Alignment and Search Tool) (http://meme.sdsc.edu/meme/meme.html) [183, 184]software was then used to look for related groups within the HCMp dataset. MEME/MAST software is especially useful for discovering patterns where little or nothing isknown in advance. We found a total of nine groups, with groups 1 and 2 containing mostof the HCMp’s (25 proteins), but with diverse motif structure within each group, withHCNCp in group 1. In contrast, the rest of the groups (groups 3-6, TMK-like, VSP-likeand EGF-like) were remarkably similar to each other within each classification. Theimportance and function of these 9 groups are presently undetermined, but suggestdistinct roles and functions bases on sequence similarity, with the possibility of otherHCNCp’s existing. Proprotein convertase 6B (PC6B) is thought to be involved with theendoproteolytic processing of precursor proteins [185], and contains the repeatedcysteine-rich domain: Cx2Cx3Cx2Cx5-7Cx2Cx10-15Cx3C. This domain is thought to involve

21
the stabilization or intracellular localization of an endoproteinase [46]. A regularexpression was constructed from the published consensus sequence and interestingly;HCNCp had 4 of these characteristic motifs. The bioinformatic methods and analysissoftware used are not only freely available, but also extremely useful, in helping tounderstand what is either presently experimentally intractable or too labor-intensive topursue otherwise.
7.3 PAPER III
The onset of differentiation in many cell types is associated with arrest of the cell cycle,suggesting that cell cycle proteins influence the transition into the differentiated state[75]. Environmental stresses directly affect the various stages of the cell cycle and thecheckpoints [186]. Checkpoints are control mechanisms that ensure accurate cell divisionbefore progression into the next cell cycle phase, and can exist at every point in the cellcycle. A special checkpoint is called the restriction point, where a shift occurs betweenproliferative and quiescent states at a specific time in the cell cycle. In other cells thiscritical release event usually occurs in G1. This allows cells to retain viability by shiftingto a minimal metabolism during differentiation or when conditions are suboptimal forgrowth [187]. In Paper III we describe development and validation of a synchronizationmethod using aphidicolin and the use of that synchronization to determine adifferentiation restriction point in Giardia.
Aphidicolin is a reversible inhibitor of eukaryotic nuclear DNA replication. It blocks thecell cycle at early S-phase, and is a specific inhibitor of eukaryotic DNA polymerase Aand D. We first determined that 5 mg ml-1 was the minimal dose of aphidicolin needed toinduce cell growth arrest in a 24 h exponentially growing population. Flow cytometryanalysis was then used to estimate the length of time in each different cell cycle stage.Most untreated trophozoites (70-80%) from an exponentially growing population were inG2/M/cytokinesis, as has previously been reported [76]. Based on flow cytometryanalysis of untreated cells and a 6 h generation time (Reiner, unpublished) we estimatedthe time the cells were in each stage as: G1 (0.7h), S (1.3h) and G2/M/cytokinesis (4h).Successful synchronization is dependent upon release after arrest in G1/S and we did thatby replacing the aphidicolin-containing medium with fresh growth medium. We arrestedtrophozoites for 6 h with 5 mg ml-1 aphidicolin in a stop-release synchronizationexperiment. After release many cells rapidly (≤ 0.5h) entered S phase and the DNAcontent increased from 4N (G1) to 8N (G2) between 1-2 h. By 3 h post-release a 4Nsubpopulation appeared, indicating that cell division had occurred. The relative size ofthe 4N peak increased until the 4 h time-point and decreased again as the synchronizedpopulation re-entered S phase. It is unlikely that aphidicolin caused much cellulardamage, since virtually the entire G1-arrested population resumed replication, andsynchrony was maintained during one whole cell cycle.
The WB-C6 isolate belongs to assemblage A and is the strain used for genomic andtranscriptomic studies, but does not infect mice. Assemblage B isolates have been used inan experimental human and [188], animal infection studies, and are found in naturaloutbreaks [189-191]. So we followed the same protocol using assemblage B (GSM83-H7) with the experimentally determined longer 10 h generation time and the same

22
aphidicolin concentration (5 mg ml-1) to synchronize and successfully release from G1/Sarrest. This demonstrates the utility of this synchronization method for both major humangenotypes.
We next wanted to evaluate both synchrony and gene expression in aphidicolin-treatedpopulations, so we bioinformatically identified orthologs of cell cycle genes in Giardia tofind candidates for analysis by real-time quantitative PCR (QPCR). We identified thetotal number of Giardia cell cycle orthologs (Reiner, Svard, Gillin, unpublished data)using reciprocal BLAST (E-value ≤ e-6) best-match analysis of 4 model organism(human, plant, budding yeast and fission yeast). This cell cycle ortholog dataset was thenused to identify Giardia orthologs of 2,100 professionally curated transcriptionallyregulated cell cycle genes (http://www.cbs.dtu.dk/cellcycle/) from the same modelorganisms [192]. The authors of this seminal study classified the transcriptionallyregulated cell cycle genes into approximately 800 orthologous groups. Strikingly, only 5orthologous groups of cell cycle genes were periodically expressed in all 4 modelorganisms; the mitotic cyclins, three groups of histone proteins (H2A, H2B and H4) andsubunits of the ribonucleotide-diphosphate reductase (RNR) complex [192]. Weidentified a total of 217 putative transcriptionally regulated genes in Giardia, but only 8from the four orthologous core cell cycle groups, since Giardia lacks the RNR pathway[193]. The unidirectional progression from one cell cycle phase to the next is due to theirreversible nature of mitotic cyclin destruction. The transition from G2 phase to mitosisis controlled by cyclin dependent kinase Cdk1 and cyclin B1 [194]. Cyclin B is normallyup-regulated in the late G2 stage of the cell cycle, promotes G2/M transition, and is thentargeted for ubiquitination and proteasomal degradation during mitosis [195]. Webioinformatically identified and confirmed a Giardia cyclin B ortholog (gCycB;GL50803_3977), using phylogenetic analysis (protein maximum likelihood alignment)comparing it to cyclins in 38 other species. We used glycyl tRNA synthetase(GL50803_9011), which showed constant expression throughout the cell cycle, as anendogenous control for real-time QPCR analysis. By comparing the ratio of gCycB toglycyl tRNA synthetase after release from aphidicolin arrest, we found that gCycBexpression gradually increased reaching a maximum at 3 h corresponding to G2, anddecreased again with entry into G1/cytokinesis after 4 h. The three putativetranscriptionally regulated cell cycle histone genes: H2A (GL50803_14256), H2B(GL50803_121045) and H4 (GL50803_135001 were upregulated early after release,similar to the large number of early S-phase cells, observed at 0.5 and 5.5 h in the flowcytometry analysis. This confirms that periodic cell cycle regulation is conserved in, andis part of, Giardia’s relatively simple cell cycle machinery. Using a comparativebioinformatic approach, we now putatively have a dataset of Giardia’s transcriptionallyregulated cell cycle genes. It will be quite informative to see how many of our predictionsare correct (or even close), after our synchronized microarray study is completed. (Troell,Reiner, Svard unpublished).
Previous studies suggested that commitment to encystation occurs via exit from aputative cell cycle restriction point in G2 [76]. To test the hypothesis that encystationefficiency in a synchronized population should be highest immediately after therestriction point is passed, we used an easily measured marker for encystation, thebiosynthesis of ESV’s, the prerequisite for Giardia cyst wall formation. The highestproduction of ESVs was observed when encystation was initiated approximately 3 h post

23
release from aphidicolin arrest. This corresponds to mid/late G2 phase and suggests thatthe encystation restriction point is located in the G2 stage of the giardial cell cycle. This isan important result, not only biologically but also technically. First, because itstrengthens the initial report that Giardia makes the final decision to either complete itscell cycle or exit into dormancy late in G2 [76]. Secondly, it provides the necessary toolsfor further analyses of this important differentiation.
7.4 PAPER IV
In other cells the basal body is central to the cell cycle and differentiation [42, 48, 49,57]. In Paper IV we integrate the data obtained using high-throughput organelle andaffinity proteomics with genomic and transcriptional data obtained during differentiationusing bioinformatic tools to build a basal body interolog network. We tested thehypothesis that the basal bodies are the cellular control center during bothdifferentiations.
Giardia cytoskeletons including basal bodies were isolated from non-synchronizedcultures of mostly interphase (2-4% in mitosis) trophozoites by detergent extraction.After sonication and Dnase treatment, the cytoskeletons were pelleted and then separatedusing sucrose density gradient (20-65%) centrifugation and fractions collected. Thefractions with the highest concentration of basal bodies, identified using centrin antibody,were analyzed by MudPIT. Individual peptides were matched to proteins in the Giardiagenome. Three independent analyses detected a total of 23,402 peptides, corresponding to310 proteins (identified by at least two peptides) and 358 proteins by a single peptidesignal.
Giardia protein homologs were identified (BLASTp; E-value ≤ e-10) and validated usingpublished data. They include: Giardia (7 basal body proteins), centrosome, centriole orbasal body proteins from other organisms (133), human spindle proteins (13),flagella/cilia (21 proteins) and proteomic and immunolocalization studies (24 proteins).There were 98 proteins identified without any sequence similarity and considered uniqueto Giardia. We confirmed that 7 of these Giardia-specific proteins are indeed in thebasal body by epitope-tagging and immunolocalization. This validates our purificationand analyses. Of these, 3 localized only to the basal bodies while the remainder alsolocalized to flagella and/or paraflagellar rods and the median body. The localization ofbasal body proteins to these mysterious organelles provides valuable insights into theirrole in giardial behavior.
Post-genomic Giardia biology involves converting genome-scale and high-throughputtranscriptional and proteomic data into systems-level insights. But working at this scalerequires biologically plausible simplifying assumptions, and a robust verificationstrategy, especially with a divergent protozoan like Giardia. In network inferencemodels, three broad types of validation are used: experimental, literature-driven curationand computational cross-validation [196]. Due to the paucity of giardial geneticapproaches, only the latter is a viable option. But with a completed genome, adifferentiation transcriptome and basal body organelle proteome, To understand thefunctional relationships of Giardia’s basal body proteome, in our further analyses, we

24
included: (1) basal body-enriched proteins identified by ≥ 2 peptides), (2) proteinsdetected by only single peptides that were cross-validated to other basal body proteomes,(3) immunologically (ELISA or IFA) validated (4) and known Giardia basal bodyproteins. We first identified human orthologs of these giardial basal body proteins usingphylogenomic analysis. The use of functional orthology allowed us to construct a highconfidence protein-protein interaction network (613 proteins and 3,023 interactionedges). To generate a higher level organizational model, the interaction edges in theorthologous interactome, were clustered into functional categories followed by manualrefinement. The multiple human homologs of each Giardia protein is collapsed into acorresponding single Giardia node, and the overall interaction edges are retained, togenerate a Giardia interolog network model (interactome based on functional homologs).The resulting network shows a striking multipartite organization with clusters showingtwo major levels of organization. At the lower level there are eight protein clusters withhigh clustering coefficients (61/93 edges among 49 nodes) having strongly correlated GO(gene ontology) functional annotation reminiscent of molecular machines: signalrecognition (CaM, Ras GTPases), signal transduction (NEK, CDK [cyclin-dependentkinase], PKA, protein phosphatases), and effector complexes (cytoskeletal motorproteins, and protein biosynthesis apparatus). The higher level of organization representscommunication channels or functional interdependencies among specialized clusters.Thus, the interolog model reveals a hitherto unappreciated level of complexity in thefunction of Giardia basal bodies. The Giardia interolog model is a minimal model, as itincludes only those proteins with human homologs, and the absence of specific proteinsin the interolog model does not exclude their functional role in the basal body.
While the interolog model is static, Giardia is a highly dynamic organism that respondsappropriately to a variety of external signals. Both encystation and excystation entailglobal changes in cell shape, motility, division and metabolism that must be coordinated.To address whether the expression of genes encoding basal body proteins changes duringdifferentiation, we measured their mRNA expression levels during growth, encystationand excystation by SAGE. Forty-seven percent (175/369 genes) of the basal bodyproteins had a SAGE tag. There were 118 transcripts that significantly changed duringdifferentiation (encystation/excystation) of these 61% were upregulated in encystationand 31% during excystation.
When SAGE expression data was overlaid onto the basal body interolog network, themodel predicts that during encystation protein biosynthesis is upregulated and duringexcystation signaling and cytoskeletal genes are upregulated. This hypothetical networkprediction is quite satisfying because it is congruent with the biology of differentiation.Specifically, new pathways for cyst wall protein synthesis and transport are induced. Wehave shown that Giardia basal body signaling proteins are required for and areupregulated in cysts and during excystation. The increase in cytoskeletal proteins reflectstheir need for cytokinesis and motility during excystation. The model therefore supportsthe centrality of basal bodies in regulating differentiation.
CaM localizes exclusively to the giardial basal bodies (Paper 1). To independentlycorroborate the interolog model, we used CaM as the bait in an affinity purificationexperiment. Fifty-four proteins from cellular extracts bound to CaM-conjugatedSepharose in a calcium dependent manner (EGTA elutable), of these, 10 were found in

25
our basal body proteome and validated by comparative proteomic analysis. In support ofthe interolog model, these 10 proteins represented five major functional groups, with 4 ofthem known to immunolocalize to Giardia basal bodies. The lack of direct interactionedges between most of the purified proteins and CaM in the interolog model suggests thatCaM-binding by these proteins may be indirect. Alternatively, in Giardia these proteinsmight bind calmodulin through uncharacterized motifs. Ribosomal proteins are especiallyprominent in basal body proteomic analyses in other organisms and are often consideredcontaminants. However, the multiple interactions between these proteins and proteins ofother functional groups, and our finding that several such proteins co-purify withcalmodulin, suggest that they may be legitimate basal body components. The dynamicnature and complex composition of Giardia’s basal bodies captured in the interologmodel further strengthens the hypothesis in Paper I suggesting that basal bodies are acommunication center regulating Giardia differentiation (Fig. 1). These findings are alsoconsistent with previous inhibitor studies of basal body proteins during excystation.Together these findings demonstrate an unappreciated level of sophistication of basalbody signaling during protozoan differentiation and flagellar biogenesis and potentiallyserve as a generalized model of cellular dormancy and awakening.
Figure 1. The basal body interolog network. The network will be used for hypothesis generation andexperimental verification. The functions of the basal bodies during encystation and excystation arepredicted using SAGE data. Ongoing experiments will use synchronized populations for isolating basalbodies for proteomics and cell cycle microarrays.

26
8 CONCLUSIONS
Systems biology studies complex interactions in biological systems, from the perspectiveof integration rather than reduction. It involves discovering emergent properties that arisefrom global interactions in a biological system. From the first time I heard about it, thissubject has fascinated me, and it has been a true joy during my doctoral studies to help“assemble” a Giardia network model. The challenge has been to acquire enough skills tocomputationally interpret the biological “wiring” of a supposedly simple cell likeGiardia, which in actuality contains an unappreciated depth of complexity. Fortunately,during the last 5 years, there has been an explosion of Giardia-specific “omic” data, andexpert collaborators, willing to attempt such an ambitious undertaking. The increasingintegration of well-planned empirical research, with the sophistication and speed inherentto in silico research, will continue to provide a deep well to draw from, for future data-driven Giardia research.
In this thesis the roles of Ca2+ signaling, homeostasis and transduction during excystationwere examined and a cellular control center hypothesized (Paper I), and suggested(Paper IV), to be localized to Giardia’s 8 basal bodies. The role of Ca2+ signaling and thefunction of the basal bodies throughout the cell cycle remains unexplored, but with aworking synchronization method (Paper III), investigations are now feasible.
The discovery of a differentiation-specific cysteine-rich protein family (Paper II) at firstappeared at first to be “off the beaten” thesis track, but that, interestingly, remains to bedemonstrated. We will soon know if there are any cell cycle-specific members of theHCMp family, after the results of the recently completed synchronized microarrayexperiments are analyzed (Troell, Reiner, Svard, unpublished). At the very least, onenovel invariant cyst protein was discovered, which curiously localizes to the cyst body.Further studies are needed, using fluorescently-tagged HCNCp during excystation, to beable to definitively understand if it is truly an excyzoite-specific protein. Other futurestudies needed concern the role of the HCMp homologs from other species. Interestingly,one of those HCMp containing species Spironucleus salmonicida, is Giardia’s closerelatives and can be cultivated at 40 C (Svard, Andersson unpublished), and is anexcellent candidate for future in vitro differentiation studies.
The understanding of Giardia’s encystation restriction point (Paper III) is in its infancy.We used ESV’s as our marker for encystation, but synchronizing cyst formation, couldpossibly be used for increasing the presently low levels of excyzoites seen during in vitroexcystation. In addition, since excystation itself is a natural synchronization, furtherstudies using flow cytometry could be implemented to determine where excyzoites re-enter the cell cycle after awakening from dormancy.
The temporal phase of the cell cycle where data is collected from is a key determinant ofprotein interactions [197]. One drawback of our basal body interolog model (Paper IV) isusing interphase trophozoites for the isolating basal bodies. At least one Giardiasignaling protein, aurora kinase localizes to the basal bodies during mitosis [52], andanalyzing basal bodies from synchronized trophozoites will increase the proteomicsensitivity of other important temporally expressed proteins. Following our initial

27
approach of predicting basal body function during differentiation using SAGE dataoverlaid onto the basal body network (Paper IV), experiments are in progress, usingsynchronized cells and Giardia microarrays to globally identify cell cycle-specificproteins at each phase of the Giardia cell cycle (Troell, Reiner, Svard unpublished). Wecan then use the cell cycle transcriptome data overlaid onto our current model to predictkey cell cycle proteins for further verification using QPCR. Equally important as thephase of the cell cycle, is the physical state of the cytoskeleton, in restricting potentialinteractions in cellular networks [165]. It could be that the tripartite structure seen in theinterolog model reflects the interphase localization of basal bodies to the cytoskeleton.Using basal body proteins from synchronized populations at all stages of the cell andlifecycle will be quite informative for understanding cytoskeletal remodeling and forrefining our network model. We are also in the process of constructing a Giardia cellcycle network model from microarray data to eventually look at Giardia’s special cellcycles during differentiation. The activation of signal transduction pathways causestranscription factors to induce global expression programs that direct cells along distinctdevelopmental pathways [197]. Another global approach would be to use a genome-widebinding assays that combines chromatin immunoprecipitation (ChIP) with DNAmicroarray technology [145]. These could be used to look for transcription factorscontrolling Giardia’s dramatic responses to physiomimetic stimuli. It is hoped that,understanding the relation of the cell cycle to differentiation at a network level, in a“simple” protozoan like Giardia, may shed deeper insights into the mechanisms of cellcycle arrest and awakening from dormancy in other complex eukaryotic cells.

28
9 ACKNOWLEDGEMENTS
I want to express my deepest gratitude and thankfulness to all those (listed below)who have been there to make this dream a reality, whether they knew it or not:----------------------------------------------------------------------------------------------------------To My Mentors:Fran – To my other job interviewer--If it’s true “iron sharpens iron”, then I should be arazor blade by now. Too little time and not enough space to express my gratitude forthe three decades of hard work, good science and lifelong friendship.Staffan - For initiating, facilitating and making me realize I could do this. For thehumor, the enthusiasm, deep creativity and classic Swedish hospitality. I will always begrateful!----------------------------------------------------------------------------------------------------------To My Teachers:Daniel Burrows- my first science teacher, Ralph Scorpio- taught me the onlybiochemistry I ever learned.Michael Blum and John Teske- who taught me pathologyNIH :Roland Faulkner, Louis Diamond-Thanks for giving a rookie a chance, David Keister-Who put the DK in TY, Eugene Weinbach- biochemist extraordinaire, MichaelGottlieb-never without a smile, Allen Sher, Ted Mattern, Alan Cheever,To my Bioinformatics InstructorsAndrew McArthur –someday I’m going to write some Perl || Mitch Sogin-hope thisisn’t too folksy?Hilary Morrison-thanks for the genome, Michael Cipriano- my computer hero.Gene Owens, Mihai Pop, John Quackenbush , Gary Owens, Alex BatemanUCSD: To My Giardia Colleagues:Past: Shayne “The Clash” Boucher, Steve Aley-thanks for not telling about NewOrleans.Siddhartha Das-who can eat the hottest food and still laugh, Gary “G-Man” Mezarros-Mr. Ca2+
Present: Barb “Babs” Davids-now I can go to Bronx again, Tineke “BelgianProt”Lauwaet-back to the networks, Charles Davis-who taught me how its done, Herndon“Big Bro” Douglas, Mike Gault, Scott Herdmann, ChooMan, Ken Hirata, LynetteCorbeil, Rachel Schreier, Don GuineyDavid Bailey, ChooMan, Cassie, Julia and Cynthia.To My People:SouthBay-Too many true believers to mention --I love you all!!Yard 5- “This ones for you!”James Robins-who showed me the way back!To My American Family:Aunt Sylvia & Uncle Mac-who asked me the toughest Giardia questions ever?Rosella Reiner- My lifelong angelKarl & Anna Houmann-- will you still talk to me, even if its not a Danish degree?Lars “RoadTrip” Houmann, Julie R., KeeKee, Cammie, and Peter “McGyver”Houmann- you guys rock! Brian, Margo, Reese, Allie Page- When are we going tocheck out that new cabin?

29
Thad & Amy, Russ & Jen, Eric & Lisa, Bruce “EasyRider” Hanscom , OuidaHanscom,Big Al & Carol Mae – Is it warm enough to visit yet?Mike & Chris, -Love you all, even if you are Patriot fansTo my Swedish family:Tord and Eva- for your hospitality, graciousness and Midsommer memoriesEva, Kalle and Johan–for my very first memories of Uppsala.Thank-you for taking me in so warmly. When you coming back to visit?Anna, Moa and Vera- for my very first memories of Stockholm. Next time the steakwill be perfect. Daniel-Thank-you for all the crazy times, for the smiles, the soccerriots, the songs and of course, the science-you truly have the gift of landing on your feetand define for me swedishness.To the Blue Mountain Boys: Steve “Little Buddy” Weider—thanx for getting me myNIH job.Randy”TR7” Gray, Richard “StrongMan” KramerFor getting me thru the tuff years:Sharon Reed- For Lauren and Gail--can never thank you enough. Jim & Annette Neal --for being there thru it all. Ed & Karen Bryan--Ed, so glad I never had to go toe-to-toewith you.Scottie- for that time at Coasters! Terri & Doug “Pushing Tin” Cohee –for getting meoff the street and on my feet. Sheryl & Jim “the Captain” Miller- for teaching me howto sail, on more than just the water.Henry & Syrini De Silva-for all the spicy hot food, warm hearts and deep smiles.Leo -for taking care of Lauren & Cynthia -for taking care of me.My California Bike Crew: Mark “Mr.Clean” White, Joseph “MLK” Lewis, and Burt“Barrack” NorrisMark-thanks for the constant support and friendship. We’re going up (:---------------------------------------------------------------------------------------------To the “Bad Boyz of Giardia” (in order of service)1) Per Hagblom#- One tuff ‘Ol Boy2) Staffan Svard#- Loves wide-mouth Mickies, Manchester United—prefers SauceBéarnaise.3) Daniel Palm#- Olympic torch protester (2004), Hammarby hooligan and Béarnaisesauce aficionadoKatarina Roxstrom-Lindquist*, Rolf “G2/M” Bernander *, Johan “Crosscheck”Ankarklev*Karin “InSynch” Troell*, Hanna Skarin*, Uffe Ribacke*-your finally working with theright parasite.
*Eligible to become members in good standing after:1) Finishing one tour of duty in San Diego.2) Valid receipt from either Phil’s BBQ, Bronx Pizza or El Cuervo.3) Preparing the perfect Béarnaise sauce.
-------------------------------------------------------------------------------------------------

30
To my parents: Harold and Ellen- Thank-you from the bottom of my heart for nevergiving up on me.And for the unconditional love- could not even imagine where I would be without it. Ilove you!To my children: Jeremy, Sonja, Lauren, Alex, Julie otherwise known as:
SurfKing, Jimbo, BabyDoll, Dinkman and Cameron Diaz and their other-halves: Matt “Yes sir” Anderson and Andrew StraujaDreams really do come true ---Never give up! I love you all.Big Al –now it’s your turn to shine, never look back. You’re the one they can’t beat!BabyDoll-I am so proud of you and happy for the place you’re at. You are truly anoriginal!--------------------------------To my New York Lady:Gail Ellen “Hanscom” Reiner--From a summer kiss 35 ago-- until now, you werealways on my mind, in my heart, and of my soul. Could not have done this without you.All my love forever!

31
10 REFERENCES
1. Wood, J.D., Neuropathophysiology of functional gastrointestinal disorders.World J Gastroenterol, 2007. 13(9): p. 1313-32.
2. Dizdar, V., O.H. Gilja, and T. Hausken, Increased visceral sensitivity inGiardia-induced postinfectious irritable bowel syndrome and functionaldyspepsia. Effect of the 5HT3-antagonist ondansetron. NeurogastroenterolMotil, 2007. 19(12): p. 977-82.
3. Farthing, M.J., Host-parasite interactions in human giardiasis. Q J Med, 1989.70(263): p. 191-204.
4. de Souza, W., A. Lanfredi-Rangel, and L. Campanati, Contribution ofmicroscopy to a better knowledge of the biology of Giardia lamblia. MicroscMicroanal, 2004. 10(5): p. 513-27.
5. Rendtorff, R.C., The experimental transmission of human intestinal protozoanparasites. II. Giardia lamblia cysts given in capsules. Am J Hyg, 1954. 59(2):p. 209-20.
6. Lebbad, M., et al., Dominance of Giardia assemblage B in Leon, Nicaragua.Acta Trop, 2008.
7. Wielinga, C.M. and R.C. Thompson, Comparative evaluation of Giardiaduodenalis sequence data. Parasitology, 2007. 134(Pt 12): p. 1795-821.
8. Simon, M.C. and H.J. Schmidt, Antigenic variation in ciliates: antigenstructure, function, expression. J Eukaryot Microbiol, 2007. 54(1): p. 1-7.
9. Gillin, F.D., et al., Isolation and expression of the gene for a major surfaceprotein of Giardia lamblia. Proc Natl Acad Sci U S A, 1990. 87(12): p. 4463-7.
10. Deak, J.C. and F.P. Doerder, Sequence, codon usage and cysteine periodicity ofthe SerH1 gene and in the encoded surface protein of Tetrahymenathermophila. Gene, 1995. 164(1): p. 163-6.
11. Kovacs, J.A., et al., Multiple genes encode the major surface glycoprotein ofPneumocystis carinii. J Biol Chem, 1993. 268(8): p. 6034-40.
12. Morrison, H.G., et al., Genomic minimalism in the early diverging intestinalparasite Giardia lamblia. Science, 2007. 317(5846): p. 1921-6.
13. Nash, T.E., J.W. Merritt, Jr., and J.T. Conrad, Isolate and epitope variability insusceptibility of Giardia lamblia to intestinal proteases. Infect Immun, 1991.59(4): p. 1334-40.
14. Aley, S.B. and F.D. Gillin, Giardia lamblia: post-translational processing andstatus of exposed cysteine residues in TSA 417, a variable surface antigen. ExpParasitol, 1993. 77(3): p. 295-305.
15. Nash, T.E., et al., Frequency of variant antigens in Giardia lamblia. ExpParasitol, 1990. 71(4): p. 415-21.
16. Nash, T.E., et al., Variant-specific surface protein switching in Giardia lamblia.Infect Immun, 2001. 69(3): p. 1922-3.
17. Svard, S.G., et al., Differentiation-associated surface antigen variation in theancient eukaryote Giardia lamblia. Mol Microbiol, 1998. 30(5): p. 979-89.
18. Lopez-Rubio, J.J., L. Riviere, and A. Scherf, Shared epigenetic mechanismscontrol virulence factors in protozoan parasites. Curr Opin Microbiol, 2007.10(6): p. 560-8.
19. Kulakova, L., et al., Epigenetic mechanisms are involved in the control ofGiardia lamblia antigenic variation. Mol Microbiol, 2006. 61(6): p. 1533-42.
20. Emanuelsson, O., et al., Locating proteins in the cell using TargetP, SignalPand related tools. Nat Protoc, 2007. 2(4): p. 953-71.

32
21. Tilney, L.G. and M.S. Tilney, The cytoskeleton of protozoan parasites. CurrOpin Cell Biol, 1996. 8(1): p. 43-8.
22. Elmendorf, H.G., S.C. Dawson, and J.M. McCaffery, The cytoskeleton ofGiardia lamblia. Int J Parasitol, 2003. 33(1): p. 3-28.
23. Pathuri, P., et al., Apo and calcium-bound crystal structures of Alpha-11giardin, an unusual annexin from Giardia lamblia. J Mol Biol, 2007. 368(2): p.493-508.
24. Crossley, R. and D.V. Holberton, Characterization of proteins from thecytoskeleton of Giardia lamblia. J Cell Sci, 1983. 59: p. 81-103.
25. Crossley, R. and D.V. Holberton, Selective extraction with Sarkosyl andrepolymerization in vitro of cytoskeleton proteins from Giardia. J Cell Sci,1983. 62: p. 419-38.
26. Morgan, R.O. and M.P. Fernandez, Molecular phylogeny of annexins andidentification of a primitive homologue in Giardia lamblia. Mol Biol Evol,1995. 12(6): p. 967-79.
27. Morgan, R.O. and M. Pilar Fernandez, Distinct annexin subfamilies in plantsand protists diverged prior to animal annexins and from a common ancestor. JMol Evol, 1997. 44(2): p. 178-88.
28. Weber, K., et al., SF-assemblin, the structural protein of the 2-nm filamentsfrom striated microtubule associated fibers of algal flagellar roots, forms asegmented coiled coil. J Cell Biol, 1993. 121(4): p. 837-45.
29. Nohria, A., R.A. Alonso, and D.A. Peattie, Identification and characterizationof gamma-giardin and the gamma-giardin gene from Giardia lamblia. MolBiochem Parasitol, 1992. 56(1): p. 27-37.
30. Moss, S.E. and R.O. Morgan, The annexins. Genome Biol, 2004. 5(4): p. 219.31. Weiland, M.E., et al., Annexin-like alpha giardins: a new cytoskeletal gene
family in Giardia lamblia. Int J Parasitol, 2005. 35(6): p. 617-26.32. Crossley, R. and D. Holberton, Assembly of 2.5 nm filaments from giardin, a
protein associated with cytoskeletal microtubules in Giardia. J Cell Sci, 1985.78: p. 205-31.
33. Mitchell, D.R., The evolution of eukaryotic cilia and flagella as motile andsensory organelles. Adv Exp Med Biol, 2007. 607: p. 130-40.
34. Scholey, J.M., Intraflagellar transport motors in cilia: moving along the cell'santenna. J Cell Biol, 2008. 180(1): p. 23-9.
35. Braiman, A., O. Zagoory, and Z. Priel, PKA induces Ca2+ release andenhances ciliary beat frequency in a Ca2+-dependent and -independentmanner. Am J Physiol, 1998. 275(3 Pt 1): p. C790-7.
36. Gadelha, C., et al., Cryptic paraflagellar rod in endosymbiont-containingkinetoplastid protozoa. Eukaryot Cell, 2005. 4(3): p. 516-25.
37. Ghosh, S., et al., How Giardia swim and divide. Infect Immun, 2001. 69(12): p.7866-72.
38. Crossley, R., et al., Immunocytochemical differentiation of microtubules in thecytoskeleton of Giardia lamblia using monoclonal antibodies to alpha-tubulinand polyclonal antibodies to associated low molecular weight proteins. J CellSci, 1986. 80: p. 233-52.
39. Benchimol, M., et al., Visualization of the funis of Giardia lamblia by high-resolution field emission scanning electron microscopy--new insights. J StructBiol, 2004. 147(2): p. 102-15.
40. Holberton, D.V., Attachment of Giardia-a hydrodynamic model based onflagellar activity. J Exp Biol, 1974. 60(1): p. 207-21.

33
41. Nohynkova, E., P. Tumova, and J. Kulda, Cell division of Giardia intestinalis:flagellar developmental cycle involves transformation and exchange of flagellabetween mastigonts of a diplomonad cell. Eukaryot Cell, 2006. 5(4): p. 753-61.
42. Lauwaet, T., et al., Encystation of Giardia lamblia: a model for other parasites.Curr Opin Microbiol, 2007. 10(6): p. 554-9.
43. Coggins, J.R. and F.W. Schaefer, 3rd, Giardia muris: scanning electronmicroscopy of in vitro excystation. Exp Parasitol, 1984. 57(1): p. 62-7.
44. Kilburn, C. and M. Winey, Basal bodies. Curr Biol, 2008. 18(2): p. R56-7.45. Sagolla, M.S., et al., Three-dimensional analysis of mitosis and cytokinesis in
the binucleate parasite Giardia intestinalis. J Cell Sci, 2006. 119(Pt 23): p.4889-900.
46. Holberton, D.V. and A.P. Ward, Isolation of the cytoskeleton from Giardia.Tubulin and a low-molecular-weight protein associated with microribbonstructures. J Cell Sci, 1981. 47: p. 139-66.
47. Benchimol, M., The nuclei of Giardia lamblia--new ultrastructuralobservations. Arch Microbiol, 2005. 183(3): p. 160-8.
48. Reiner, D.S., et al., Calcium signaling in excystation of the early divergingeukaryote, Giardia lamblia. J Biol Chem, 2003. 278(4): p. 2533-40.
49. Abel, E.S., et al., Possible roles of protein kinase A in cell motility andexcystation of the early diverging eukaryote Giardia lamblia. J Biol Chem,2001. 276(13): p. 10320-9.
50. Gibson, C., et al., Functional characterisation of the regulatory subunit ofcyclic AMP-dependent protein kinase A homologue of Giardia lamblia:Differential expression of the regulatory and catalytic subunits duringencystation. Int J Parasitol, 2006. 36(7): p. 791-9.
51. Lauwaet, T., et al., Protein phosphatase 2A plays a crucial role in Giardialamblia differentiation. Mol Biochem Parasitol, 2007. 152(1): p. 80-9.
52. Davids, B.J., et al., Giardia lamblia aurora kinase: A regulator of mitosis in abinucleate parasite. Int J Parasitol, 2008. 38(3-4): p. 353-69.
53. Keller, L.C., et al., Proteomic analysis of isolated chlamydomonas centriolesreveals orthologs of ciliary-disease genes. Curr Biol, 2005. 15(12): p. 1090-8.
54. Kilburn, C.L., et al., New Tetrahymena basal body protein components identifybasal body domain structure. J Cell Biol, 2007. 178(6): p. 905-12.
55. Andersen, J.S., et al., Proteomic characterization of the human centrosome byprotein correlation profiling. Nature, 2003. 426(6966): p. 570-4.
56. Reinders, Y., et al., Identification of novel centrosomal proteins inDictyostelium discoideum by comparative proteomic approaches. J ProteomeRes, 2006. 5(3): p. 589-98.
57. Ellis, J.G.t., M. Davila, and R. Chakrabarti, Potential involvement ofextracellular signal-regulated kinase 1 and 2 in encystation of a primitiveeukaryote, Giardia lamblia. Stage-specific activation and intracellularlocalization. J Biol Chem, 2003. 278(3): p. 1936-45.
58. Meng, T.C., et al., Immunolocalization and sequence of caltractin/centrin fromthe early branching eukaryote Giardia lamblia. Mol Biochem Parasitol, 1996.79(1): p. 103-8.
59. Correa, G., J.A. Morgado-Diaz, and M. Benchimol, Centrin in Giardia lamblia- ultrastructural localization. FEMS Microbiol Lett, 2004. 233(1): p. 91-6.
60. Nohynkova, E., et al., Localization of gamma-tubulin in interphase and mitoticcells of a unicellular eukaryote, Giardia intestinalis. Eur J Cell Biol, 2000.79(6): p. 438-45.

34
61. Muller, N. and N. von Allmen, Recent insights into the mucosal reactionsassociated with Giardia lamblia infections. Int J Parasitol, 2005. 35(13): p.1339-47.
62. Erlandsen, S.L., A.P. Russo, and J.N. Turner, Evidence for adhesive activity ofthe ventrolateral flange in Giardia lamblia. J Eukaryot Microbiol, 2004. 51(1):p. 73-80.
63. Tumova, P., J. Kulda, and E. Nohynkova, Cell division of Giardia intestinalis:assembly and disassembly of the adhesive disc, and the cytokinesis. Cell MotilCytoskeleton, 2007. 64(4): p. 288-98.
64. Lechtreck, K.F. and M. Melkonian, SF-assemblin, striated fibers, andsegmented coiled coil proteins. Cell Motil Cytoskeleton, 1998. 41(4): p. 289-96.
65. Palm, D., et al., Developmental changes in the adhesive disk during Giardiadifferentiation. Mol Biochem Parasitol, 2005. 141(2): p. 199-207.
66. Piva, B. and M. Benchimol, The median body of Giardia lamblia: anultrastructural study. Biol Cell, 2004. 96(9): p. 735-46.
67. Adam, R.D., Biology of Giardia lamblia. Clin Microbiol Rev, 2001. 14(3): p.447-75.
68. Hetsko, M.L., et al., Cellular and transcriptional changes during excystation ofGiardia lamblia in vitro. Exp Parasitol, 1998. 88(3): p. 172-83.
69. Bertram, M.A., et al., A morphometric comparison of five axenic Giardiaisolates. J Parasitol, 1984. 70(4): p. 530-5.
70. Marshall, J. and D.V. Holberton, Sequence and structure of a new coiled coilprotein from a microtubule bundle in Giardia. J Mol Biol, 1993. 231(2): p. 521-30.
71. Belhadri, A., Presence of centrin in the human parasite Giardia: a furtherindication of its ubiquity in eukaryotes. Biochem Biophys Res Commun, 1995.214(2): p. 597-601.
72. Campanati, L., et al., Expression of tubulin polyglycylation in Giardia lamblia.Biol Cell, 1999. 91(7): p. 499-506.
73. Soltys, B.J. and R.S. Gupta, Immunoelectron microscopy of Giardia lambliacytoskeleton using antibody to acetylated alpha-tubulin. J Eukaryot Microbiol,1994. 41(6): p. 625-32.
74. Campanati, L., et al., Tubulin diversity in trophozoites of Giardia lamblia.Histochem Cell Biol, 2003. 119(4): p. 323-31.
75. Svard, S.G., P. Hagblom, and J.E. Palm, Giardia lamblia -- a model organismfor eukaryotic cell differentiation. FEMS Microbiol Lett, 2003. 218(1): p. 3-7.
76. Bernander, R., J.E. Palm, and S.G. Svard, Genome ploidy in different stages ofthe Giardia lamblia life cycle. Cell Microbiol, 2001. 3(1): p. 55-62.
77. Reiner, D.S., et al., Synchronisation of Giardia lamblia: Identification of cellcycle stage-specific genes and a differentiation restriction point. Int J Parasitol,2008.
78. Gillin, F.D., et al., Encystation and expression of cyst antigens by Giardialamblia in vitro. Science, 1987. 235(4792): p. 1040-3.
79. Gillin, F.D., D.S. Reiner, and S.E. Boucher, Small-intestinal factors promoteencystation of Giardia lamblia in vitro. Infect Immun, 1988. 56(3): p. 705-7.
80. Gillin, F.D., et al., Giardia lamblia: the roles of bile, lactic acid, and pH in thecompletion of the life cycle in vitro. Exp Parasitol, 1989. 69(2): p. 164-74.
81. Boucher, S.E. and F.D. Gillin, Excystation of in vitro-derived Giardia lambliacysts. Infect Immun, 1990. 58(11): p. 3516-22.
82. Gerwig, G.J., et al., The Giardia intestinalis filamentous cyst wall contains anovel beta(1-3)-N-acetyl-D-galactosamine polymer: a structural andconformational study. Glycobiology, 2002. 12(8): p. 499-505.

35
83. Faubert, G., D.S. Reiner, and F.D. Gillin, Giardia lamblia: regulation ofsecretory vesicle formation and loss of ability to reattach during encystation invitro. Exp Parasitol, 1991. 72(4): p. 345-54.
84. Reiner, D.S., M. McCaffery, and F.D. Gillin, Sorting of cyst wall proteins to aregulated secretory pathway during differentiation of the primitive eukaryote,Giardia lamblia. Eur J Cell Biol, 1990. 53(1): p. 142-53.
85. Marti, M., et al., An ancestral secretory apparatus in the protozoan parasiteGiardia intestinalis. J Biol Chem, 2003. 278(27): p. 24837-48.
86. McCaffery, J.M. and F.D. Gillin, Giardia lamblia: ultrastructural basis ofprotein transport during growth and encystation. Exp Parasitol, 1994. 79(3): p.220-35.
87. Lanfredi-Rangel, A., et al., Fine structure of the biogenesis of Giardia lambliaencystation secretory vesicles. J Struct Biol, 2003. 143(2): p. 153-63.
88. Gillin, F.D., D.S. Reiner, and J.M. McCaffery, Cell biology of the primitiveeukaryote Giardia lamblia. Annu Rev Microbiol, 1996. 50: p. 679-705.
89. Ramesh, M.A., S.B. Malik, and J.M. Logsdon, Jr., A phylogenomic inventory ofmeiotic genes; evidence for sex in Giardia and an early eukaryotic origin ofmeiosis. Curr Biol, 2005. 15(2): p. 185-91.
90. Teodorovic, S., J.M. Braverman, and H.G. Elmendorf, Unusually low levels ofgenetic variation among Giardia lamblia isolates. Eukaryot Cell, 2007. 6(8): p.1421-30.
91. Cooper, M.A., et al., Population genetics provides evidence for recombinationin Giardia. Curr Biol, 2007. 17(22): p. 1984-8.
92. Gibson, W., et al., The use of yellow fluorescent hybrids to indicate mating inTrypanosoma brucei. Parasit Vectors, 2008. 1(1): p. 4.
93. Gibson, W., et al., Analysis of a cross between green and red fluorescenttrypanosomes. Biochem Soc Trans, 2006. 34(Pt 4): p. 557-9.
94. Logsdon, J.M., Jr., Evolutionary genetics: sex happens in Giardia. Curr Biol,2008. 18(2): p. R66-8.
95. Poxleitner, M.K., et al., Evidence for karyogamy and exchange of geneticmaterial in the binucleate intestinal parasite Giardia intestinalis. Science,2008. 319(5869): p. 1530-3.
96. Heitman, J., Sexual reproduction and the evolution of microbial pathogens.Curr Biol, 2006. 16(17): p. R711-25.
97. Hegner, R. and L. Eskridge, The influence of bile salts on Giardia infection inrats. Am J Hyg, 1937. 26: p. 186-192.
98. Keister, D.B., Axenic culture of Giardia lamblia in TYI-S-33 mediumsupplemented with bile. Trans R Soc Trop Med Hyg, 1983. 77(4): p. 487-8.
99. Farthing, M.J., S.R. Varon, and G.T. Keusch, Mammalian bile promotes growthof Giardia lamblia in axenic culture. Trans R Soc Trop Med Hyg, 1983. 77(4):p. 467-9.
100. Lujan, H.D., et al., Cholesterol starvation induces differentiation of theintestinal parasite Giardia lamblia. Proc Natl Acad Sci U S A, 1996. 93(15): p.7628-33.
101. Erlandsen, S., Reduction in fecal excretion of Giardia cysts: effect of cholestasisand diet. J Parasitol, 2005. 91(6): p. 1482-4.
102. Clark, C.G. and L.S. Diamond, Methods for cultivation of luminal parasiticprotists of clinical importance. Clin Microbiol Rev, 2002. 15(3): p. 329-41.
103. Erlandsen, S.L., et al., Formation of the Giardia cyst wall: studies onextracellular assembly using immunogold labeling and high resolution fieldemission SEM. J Eukaryot Microbiol, 1996. 43(5): p. 416-29.

36
104. Manning, P., S.L. Erlandsen, and E.L. Jarroll, Carbohydrate and amino acidanalyses of Giardia muris cysts. J Protozool, 1992. 39(2): p. 290-6.
105. Erlandsen, S.L., W.J. Bemrick, and J. Pawley, High-resolution electronmicroscopic evidence for the filamentous structure of the cyst wall in Giardiamuris and Giardia duodenalis. J Parasitol, 1989. 75(5): p. 787-97.
106. Lujan, H.D., et al., Developmental induction of Golgi structure and function inthe primitive eukaryote Giardia lamblia. J Biol Chem, 1995. 270(9): p. 4612-8.
107. Lanfredi-Rangel, A., et al., Trophozoites of Giardia lamblia may have a Golgi-like structure. FEMS Microbiol Lett, 1999. 181(2): p. 245-51.
108. Mowatt, M.R., et al., Developmentally regulated expression of a Giardialamblia cyst wall protein gene. Mol Microbiol, 1995. 15(5): p. 955-63.
109. Lujan, H.D., et al., Identification of a novel Giardia lamblia cyst wall proteinwith leucine-rich repeats. Implications for secretory granule formation andprotein assembly into the cyst wall. J Biol Chem, 1995. 270(49): p. 29307-13.
110. Sun, C.H., et al., Mining the Giardia lamblia genome for new cyst wallproteins. J Biol Chem, 2003. 278(24): p. 21701-8.
111. Davids, B.J., et al., A new family of giardial cysteine-rich non-VSP proteingenes and a novel cyst protein. PLoS ONE, 2006. 1: p. e44.
112. Marti, M. and A.B. Hehl, Encystation-specific vesicles in Giardia: a primordialGolgi or just another secretory compartment? Trends Parasitol, 2003. 19(10):p. 440-6.
113. Reiner, D.S., J.M. McCaffery, and F.D. Gillin, Reversible interruption ofGiardia lamblia cyst wall protein transport in a novel regulated secretorypathway. Cell Microbiol, 2001. 3(7): p. 459-72.
114. Stefanic, S., et al., Organelle proteomics reveals cargo maturation mechanismsassociated with Golgi-like encystation vesicles in the early-diverged protozoanGiardia lamblia. J Biol Chem, 2006. 281(11): p. 7595-604.
115. Lujan, H.D. and M.C. Touz, Protein trafficking in Giardia lamblia. CellMicrobiol, 2003. 5(7): p. 427-34.
116. Knodler, L.A., et al., Novel protein-disulfide isomerases from the early-diverging protist Giardia lamblia. J Biol Chem, 1999. 274(42): p. 29805-11.
117. Touz, M.C., et al., The activity of a developmentally regulated cysteineproteinase is required for cyst wall formation in the primitive eukaryoteGiardia lamblia. J Biol Chem, 2002. 277(10): p. 8474-81.
118. Touz, M.C., et al., Identification and characterization of a novel secretorygranule calcium-binding protein from the early branching eukaryote Giardialamblia. J Biol Chem, 2002. 277(52): p. 50557-63.
119. Chavez-Munguia, B., et al., Ultrastructure of cyst differentiation in parasiticprotozoa. Parasitol Res, 2007. 100(6): p. 1169-75.
120. Sheffield, H.G. and B. Bjorvat, Ultrastructure of the cyst of Giardia lamblia.Am J Trop Med Hyg, 1977. 26(1): p. 23-30.
121. Hegner, R., Excystation in Vitro of Human Intestinal Protozoa. Science, 1927.65(1693): p. 577-578.
122. Bingham, A.K. and E.A. Meyer, Giardia excystation can be induced in vitro inacidic solutions. Nature, 1979. 277(5694): p. 301-2.
123. Rice, E.W. and F.W. Schaefer, 3rd, Improved in vitro excystation procedure forGiardia lamblia cysts. J Clin Microbiol, 1981. 14(6): p. 709-10.
124. Ward, W., et al., A primitive enzyme for a primitive cell: the protease requiredfor excystation of Giardia. Cell, 1997. 89(3): p. 437-44.
125. Lloyd, D. and P. Wallis, A Giardia feast. Trends Parasitol, 2001. 17(3): p. 115-7.

37
126. Slavin, I., et al., Dephosphorylation of cyst wall proteins by a secretedlysosomal acid phosphatase is essential for excystation of Giardia lamblia. MolBiochem Parasitol, 2002. 122(1): p. 95-8.
127. Wiesehahn, G.P., et al., Giardia lamblia: autoradiographic analysis of nuclearreplication. Exp Parasitol, 1984. 58(1): p. 94-100.
128. Adam, R.D., The Giardia lamblia genome. Int J Parasitol, 2000. 30(4): p. 475-84.
129. Hoyne, G.F., et al., The effect of drugs on the cell cycle of Giardia intestinalis.Parasitology, 1989. 99 Pt 3: p. 333-9.
130. Sandhu, H., R.C. Mahajan, and N.K. Ganguly, Flowcytometric assessment ofthe effect of drugs on Giardia lamblia trophozoites in vitro. Mol Cell Biochem,2004. 265(1-2): p. 151-60.
131. Ortega-Barria, E., et al., Growth inhibition of the intestinal parasite Giardialamblia by a dietary lectin is associated with arrest of the cell cycle. J ClinInvest, 1994. 94(6): p. 2283-8.
132. Poxleitner, M., S. Dawson, and W.Z. Cande, Cell cycle synchrony in Giardiaintestinalis cultures using nocodazole and aphidicolin. Eukaryot Cell, 2008.
133. Gillin, F.D. and L.S. Diamond, Clonal growth of Giardia lamblia trophozoitesin a semisolid agarose medium. J Parasitol, 1980. 66(2): p. 350-2.
134. Gillin, F.D. and L.S. Diamond, Entamoeba histolytica and Giardia lamblia:growth responses to reducing agents. Exp Parasitol, 1981. 51(3): p. 382-91.
135. Smith, P.D., et al., IgG antibody to Giardia lamblia detected by enzyme-linkedimmunosorbent assay. Gastroenterology, 1981. 80(6): p. 1476-80.
136. Gillin, F.D. and L.S. Diamond, Entamoeba histolytica and Giardia lamblia:effects of cysteine and oxygen tension on trophozoite attachment to glass andsurvival in culture media. Exp Parasitol, 1981. 52(1): p. 9-17.
137. Gillin, F.D. and D.S. Reiner, Attachment of the flagellate Giardia lamblia: roleof reducing agents, serum, temperature, and ionic composition. Mol Cell Biol,1982. 2(4): p. 369-77.
138. Gillin, F.D., D.S. Reiner, and P.P. McCann, Inhibition of growth of Giardialamblia by difluoromethylornithine, a specific inhibitor of polyaminebiosynthesis. J Protozool, 1984. 31(1): p. 161-3.
139. Gault, M.J., D.S. Reiner, and F.D. Gillin, Tolerance of axenically culturedEntamoeba histolytica and Giardia lamblia to a variety of antimicrobial agents.Trans R Soc Trop Med Hyg, 1985. 79(1): p. 60-2.
140. Lilly, M.A. and R.J. Duronio, New insights into cell cycle control from theDrosophila endocycle. Oncogene, 2005. 24(17): p. 2765-75.
141. Larkins, B.A., et al., Investigating the hows and whys of DNAendoreduplication. J Exp Bot, 2001. 52(355): p. 183-92.
142. Edgar, B.A. and T.L. Orr-Weaver, Endoreplication cell cycles: more for less.Cell, 2001. 105(3): p. 297-306.
143. Coppel, R.L. and C.G. Black, Parasite genomes. Int J Parasitol, 2005. 35(5): p.465-79.
144. Pinney, J.W., et al., Metabolic reconstruction and analysis for parasitegenomes. Trends Parasitol, 2007. 23(11): p. 548-54.
145. Iyer, L.M., et al., Comparative genomics of transcription factors and chromatinproteins in parasitic protists and other eukaryotes. Int J Parasitol, 2008. 38(1):p. 1-31.
146. Quackenbush, J., Extracting biology from high-dimensional biological data. JExp Biol, 2007. 210(Pt 9): p. 1507-17.
147. Cheong, R. and A. Levchenko, Wires in the soup: quantitative models of cellsignaling. Trends Cell Biol, 2008. 18(3): p. 112-8.

38
148. Gregory, T.R., Coincidence, coevolution, or causation? DNA content, cell size,and the C-value enigma. Biol Rev Camb Philos Soc, 2001. 76(1): p. 65-101.
149. Galperin, M.Y., Some bacteria degrade explosives, others prefer boilingmethanol. Environ Microbiol, 2007. 9(12): p. 2905-10.
150. Sun, C.H., et al., A novel Myb-related protein involved in transcriptionalactivation of encystation genes in Giardia lamblia. Mol Microbiol, 2002. 46(4):p. 971-84.
151. Teodorovic, S., C.D. Walls, and H.G. Elmendorf, Bidirectional transcription isan inherent feature of Giardia lamblia promoters and contributes to anabundance of sterile antisense transcripts throughout the genome. NucleicAcids Res, 2007. 35(8): p. 2544-53.
152. Seshadri, V., et al., Giardia lamblia RNA polymerase II: amanitin-resistanttranscription. J Biol Chem, 2003. 278(30): p. 27804-10.
153. Knodler, L.A., et al., Developmental gene regulation in Giardia lamblia: firstevidence for an encystation-specific promoter and differential 5' mRNAprocessing. Mol Microbiol, 1999. 34(2): p. 327-40.
154. Hochstrasser, D.F., J.C. Sanchez, and R.D. Appel, Proteomics and its trendsfacing nature's complexity. Proteomics, 2002. 2(7): p. 807-12.
155. Jung, E., et al., Proteomics meets cell biology: the establishment of subcellularproteomes. Electrophoresis, 2000. 21(16): p. 3369-77.
156. Sun, S., et al., Oncoproteomics of hepatocellular carcinoma: from cancermarkers' discovery to functional pathways. Liver Int, 2007. 27(8): p. 1021-38.
157. Ringqvist, E., et al., Release of metabolic enzymes by Giardia in response tointeraction with intestinal epithelial cells. Mol Biochem Parasitol, 2008.
158. Link, A.J., et al., Direct analysis of protein complexes using mass spectrometry.Nat Biotechnol, 1999. 17(7): p. 676-82.
159. Washburn, M.P., D. Wolters, and J.R. Yates, 3rd, Large-scale analysis of theyeast proteome by multidimensional protein identification technology. NatBiotechnol, 2001. 19(3): p. 242-7.
160. Florens, L. and M.P. Washburn, Proteomic analysis by multidimensionalprotein identification technology. Methods Mol Biol, 2006. 328: p. 159-75.
161. Paoletti, A.C., B. Zybailov, and M.P. Washburn, Principles and applications ofmultidimensional protein identification technology. Expert Rev Proteomics,2004. 1(3): p. 275-82.
162. Ideker, T. and R. Sharan, Protein networks in disease. Genome Res, 2008.18(4): p. 644-52.
163. Matthews, L.R., et al., Identification of potential interaction networks usingsequence-based searches for conserved protein-protein interactions or"interologs". Genome Res, 2001. 11(12): p. 2120-6.
164. Walhout, A.J., et al., Protein interaction mapping in C. elegans using proteinsinvolved in vulval development. Science, 2000. 287(5450): p. 116-22.
165. Barabasi, A.L. and Z.N. Oltvai, Network biology: understanding the cell'sfunctional organization. Nat Rev Genet, 2004. 5(2): p. 101-13.
166. Clapham, D.E., Calcium signaling. Cell, 2007. 131(6): p. 1047-58.167. Santella, L., E. Ercolano, and G.A. Nusco, The cell cycle: a new entry in the
field of Ca2+ signaling. Cell Mol Life Sci, 2005. 62(21): p. 2405-13.168. Periasamy, M. and A. Kalyanasundaram, SERCA pump isoforms: their role in
calcium transport and disease. Muscle Nerve, 2007. 35(4): p. 430-42.169. Inesi, G., et al., Long-range intramolecular linked functions in activation and
inhibition of SERCA ATPases. Ann N Y Acad Sci, 1992. 671: p. 32-47;discussion 48.

39
170. Andrews, S.P., et al., Total synthesis of five thapsigargins: guaianolide naturalproducts exhibiting sub-nanomolar SERCA inhibition. Chemistry, 2007. 13(20):p. 5688-712.
171. June, C.H. and P.S. Rabinovitch, Flow cytometric measurement of intracellularionized calcium in single cells with indo-1 and fluo-3. Methods Cell Biol, 1990.33: p. 37-58.
172. Zaidi, M., et al., Cytosolic free calcium measurements in single cells usingcalcium-sensitive fluorochromes. Methods Mol Biol, 1994. 27: p. 279-93.
173. Nakayama, S. and R.H. Kretsinger, Evolution of the EF-hand family of proteins.Annu Rev Biophys Biomol Struct, 1994. 23: p. 473-507.
174. Hoeflich, K.P. and M. Ikura, Calmodulin in action: diversity in targetrecognition and activation mechanisms. Cell, 2002. 108(6): p. 739-42.
175. Bernal, R.M., et al., Possible role of calmodulin in excystation of Giardialamblia. Parasitol Res, 1998. 84(9): p. 687-93.
176. Munoz, M.L., et al., Giardia lamblia: detection and characterization ofcalmodulin. Exp Parasitol, 1987. 63(1): p. 42-8.
177. Krogh, A., et al., Predicting transmembrane protein topology with a hiddenMarkov model: application to complete genomes. J Mol Biol, 2001. 305(3): p.567-80.
178. Andersson, J.O., et al., A genomic survey of the fish parasite Spironucleussalmonicida indicates genomic plasticity among diplomonads and significantlateral gene transfer in eukaryote genome evolution. BMC Genomics, 2007. 8:p. 51.
179. McClure, M.A., T.K. Vasi, and W.M. Fitch, Comparative analysis of multipleprotein-sequence alignment methods. Mol Biol Evol, 1994. 11(4): p. 571-92.
180. Eddy, S.R., Hidden Markov models. Curr Opin Struct Biol, 1996. 6(3): p. 361-5.
181. Finn, R.D., et al., Pfam: clans, web tools and services. Nucleic Acids Res,2006. 34(Database issue): p. D247-51.
182. Eichinger, L. and A.A. Noegel, Comparative genomics of Dictyosteliumdiscoideum and Entamoeba histolytica. Curr Opin Microbiol, 2005. 8(5): p.606-11.
183. Bailey, T.L., et al., MEME: discovering and analyzing DNA and proteinsequence motifs. Nucleic Acids Res, 2006. 34(Web Server issue): p. W369-73.
184. Bailey, T.L. and M. Gribskov, Combining evidence using p-values: applicationto sequence homology searches. Bioinformatics, 1998. 14(1): p. 48-54.
185. Nakagawa, T., K. Murakami, and K. Nakayama, Identification of an isoformwith an extremely large Cys-rich region of PC6, a Kex2-like processingendoprotease. FEBS Lett, 1993. 327(2): p. 165-71.
186. Douglas, R.M. and G.G. Haddad, Genetic models in applied physiology: invitedreview: effect of oxygen deprivation on cell cycle activity: a profile of delay andarrest. J Appl Physiol, 2003. 94(5): p. 2068-83; discussion 2084.
187. Pardee, A.B., A restriction point for control of normal animal cell proliferation.Proc Natl Acad Sci U S A, 1974. 71(4): p. 1286-90.
188. Nash, T.E., et al., Experimental human infections with Giardia lamblia. J InfectDis, 1987. 156(6): p. 974-84.
189. Davids, B.J., et al., Polymeric immunoglobulin receptor in intestinal immunedefense against the lumen-dwelling protozoan parasite Giardia. J Immunol,2006. 177(9): p. 6281-90.
190. Byrd, L.G., J.T. Conrad, and T.E. Nash, Giardia lamblia infections in adultmice. Infect Immun, 1994. 62(8): p. 3583-5.

40
191. Robertson, L.J., T. Forberg, and B.K. Gjerde, Giardia cysts in sewage influentin Bergen, Norway 15-23 months after an extensive waterborne outbreak ofgiardiasis. J Appl Microbiol, 2008. 104(4): p. 1147-52.
192. Jensen, L.J., et al., Co-evolution of transcriptional and post-translational cell-cycle regulation. Nature, 2006. 443(7111): p. 594-7.
193. Baum, K.F., et al., Purine deoxynucleoside salvage in Giardia lamblia. J BiolChem, 1989. 264(35): p. 21087-90.
194. Bassermann, F., C. Peschel, and J. Duyster, Mitotic entry: a matter ofoscillating destruction. Cell Cycle, 2005. 4(11): p. 1515-7.
195. Sanchez, I. and B.D. Dynlacht, New insights into cyclins, CDKs, and cell cyclecontrol. Semin Cell Dev Biol, 2005. 16(3): p. 311-21.
196. Hayete, B., T.S. Gardner, and J.J. Collins, Size matters: network inferencetackles the genome scale. Mol Syst Biol, 2007. 3: p. 77.
197. Zeitlinger, J., et al., Program-specific distribution of a transcription factordependent on partner transcription factor and MAPK signaling. Cell, 2003.113(3): p. 395-404.