Cases of Cherubism
-
Upload
sadia-gull -
Category
Documents
-
view
70 -
download
4
Transcript of Cases of Cherubism

Int. J. Oral Maxillofac. Surg. 2005; 34: 350–356doi:10.1016/j.ijom.2004.09.006, available online at http://www.sciencedirect.com
Clinical PaperCongenital Craniofacial Anomalies
Clinicopathologic study of 24cases of cherubism
0901-5027/040350+07 $30.00/0 # 2004 International Association of Oral and Maxillofacial Surgeo
X.-M. Meng1, S.-F. Yu1, G.-Y. Yu2
1Department of Oral Pathology, PekingUniversity School of Stomatology, Beijing100081, PR China; 2Department of Oral andMaxillofacial Surgery, Peking UniversitySchool of Stomatology, Beijing 100081,PR China
X.-M. Meng, S.-F. Yu, G.-Y. Yu:Clinicopathologic study of 24 cases of cherubism. Int.J. Oral Maxillofac. Surg. 2005; 34: 350–356. # 2004 International Association ofOral and Maxillofacial Surgeons. Published by Elsevier Ltd. All rights reserved.
n
Abstract. The authors reviewed 24 cases of familial or nonfamilial cherubism.The age at onset was between 6 and 10 years. It was characterized by bilateralpainless swelling of jaws and eyes-to-heaven appearance was visible when the maxillaewere affected as well. Radiographs showed well-defined multilocular radiolucenciesand with age, thick sclerotic borders were visible. A malocclusive and abnormaldentition, worse in the mandible can be seen. Histopathologically, numerous randomlydistributed multinucleated giant cells and vascular spaces within a fibrous connectivetissue stroma with or without eosinophilic collagen perivascular cuffing were apparent.Multinucleated giant cells were positive for osteoclastic specific markers,tartrate-resistant acid phosphatase and human aV b3 integrin, 23C6. Results afterfollow-up were available for 14 cases. Of these, no treatment was carried out in fivecases, cherubism resolved (three cases) or grew slowly (two cases); curettage or surgicalcontouring was performed in seven cases, during the rapid growth of the lesions. This notonly gave good immediate results, but also arrested active growth of remnant cherubiclesions and even stimulated bone regeneration. Segmental mandibulectomy followed byreconstruction was performed in two cases with extensive lesion and the risk ofpathologic fracture of the mandible, and excellent results were obtained.
Key words: cherubism; jaw; giant cell lesion.
Accepted for publication 6 September 2004Available online 11 November 2004
Materials and methods
Cherubism was first described by JONES in19339 as ‘familial multilocular cystic dis-ease of the jaws’. He later coined thedescriptive term ‘cherubism’ when helikened the classical characteristics of fullround cheeks and upward cast of the eyesto the angelic look of the cherubs immor-talized by Renaissance art. Cherubism ischaracterized by bilateral expansion of themandible and/or the maxilla that becomesnoticeable within the first several years oflife, becomes progressively pronounceduntil puberty, with gradual involution bymiddle age. The radiographic appearanceis unique because of its diffuse, bilateral,multilocular nature. Histologically, thelesions contain numerous multinucleated
giant cells scattered throughout a fibrousconnective tissue stroma.
In China, the occurrence of cherubismhas been reported in several cases withand without familial history31,33–35. Theaim of this study is to investigate:
(1) T
he clinical and radiographic fea-tures, histopathologic appearance,biochemical markers, and the diag-nosis criteria that differentiate itfrom other giant cell lesions in thejaws.(2) T
he relationship between the naturalcourse of the disease, its severity(application of classification systems),and possible treatment modalities.(3) T
s. Pu
he results of surgical treatments withlong-term follow-up and treatmentrecommendations.
The patients consisted of 24 patients(17 were referred to our hospital, theremaining 7 were from other hospitalsin China31,34,35). Of those, 19 fulfilledthe following objective criteria of cherub-ism: (1) clinically, a painless swelling ofthe jaw, combined with (2) radiographicfindings of multilocular (rarely unilocular)radiolucencies, often very extensive, witha few irregular bony septa, and (3) patho-logic bone cavities filled in by tissue, simi-
blished by Elsevier Ltd. All rights reserved.

Clinicopathologic study 351
able 1. Distribution of age at onset inatients with cherubism
ge at onset (year) No. of cases
0–5 46–10 111–20 61–30 2>30 1
otal 24
lar to the gross and microscopic structureof a giant cell granuloma (in patients <20years of age) or fibrous tissue (in patients>20 years of age) with or without eosi-nophilic collagen perivascular cuffing. Inthe other five patients no clinically detect-able features were found and no histo-pathologic examination was performed,however the diagnosis of cherubism wasverified by radiographic examinations anda positive family history.
Routine clinical examination was per-formed in all of the patients and included(1) the facial appearance, (2) the shape ofthe buccal and lingual bone surfaces of thejaws, normal or expansive, estimated by acomparative clinical palpation of theentire bone surface of the jaws and theremaining facial bones, (3) the dentalstate, and (4) the enlargement of subman-dibular lymph nodes.
Conventional radiographic methodsincluding panoramic radiograph andWATER’s view for lesions in the maxillarysinus, were applied. The following wasobserved:
(1) L
oci and areas where there are lesions:Involvement of mandibular ascendingrami with or without maxillary tuber-osities or massive involvement of thewhole maxilla and mandible with orwithout condyles.(2) S
hape and border of the lesions: Nor-mal or the presence of multilocularradiolucencies; well-defined or unclearborder, which is sclerotic or normal.(3) B
ony cortex: Expansion, thinness,perforation or disruption.(4) T
he dental state: Normal or missingteeth, displacement, malformation, orroot resorption.radiographs were re-evaluated by a
Allradiologist.The severity grades of cherubism wereused with a supplement (in italics) fromthe SEWARD & HANKEY
23 system:
Grade I: Involvement of bilateral mandib-ular molar region and ascending rami,mandible body or mentis.Grade II: Involvement of bilateral max-illary tuberosities as well as the lesion ofGrade I, diffused whole mandible.Grade III: Massive involvement of theentire maxilla and mandible except thecondyles.Grade IV: Involvement of both jaws withcondyles.
Fig. 1. Distribution of 14 patients (*) with cherubism from six positive families. Circles identifyfemale, squares identify male and filled symbols identify affected individuals.
Biochemical analyses, such as serumcalcium, serum phosphorus, alkalinephosphatase, and parathyroid hormone
were performed in 17 patients referredto our hospital. For those with abnormalserum values, radiographic examination ofthe skeleton was performed.
Histopathologic examinations weremade by incisional biopsy or during rou-tine postoperative procedures in 19patients; the other five refused this exam-ination because classic clinical featureswere not detectable. The tissue sectionswere reviewed by a pathologist.
Histochemical and immunohistochem-ical characterization of multinucleatedgiant cells was performed. Using a com-mercially available kit (Sigma, USA), thesections were deparaffinized and stainedfor the expression of tartrate-resistant acidphosphatase (TRAP), an osteoclast-asso-ciated marker. Using naphthol AS-BIphosphate as a substrate, in the presenceor absence of 1.0 mol/L tartrate (37 8C,20–30 min), the product was reacted withFast-Garnet GBC salt. The sections werethen counterstained with hematoxylinstain. The frozen sections were fixed inpre-cooled acetone solution and stainedimmunohistochemically using an alkalinephosphatase-based method (SAP Kit,Zymed, USA) with the monoclonal anti-body against human aV b3 integrin, 23C6(Santa Cruz, USA) to determine expres-sion of the vitronectin receptor (VNR), anosteoclast associated antigen. A new fuch-
Tp
A
12
T
sin substrate, AP-Red (Zymed, USA) wasused and red staining indicated positivereactivity. The sections were then counter-stained with methyl green.
Surgical correction with removal of themain part of the lesional tissue was per-formed in 13 of the patients; 6 received aminor surgical operation for the purpose ofincisional biopsy only. No treatment wascarried out in other 5.
In the follow-up study, the clinical andradiographic information was used to esti-mate the postoperative condition or theprogress of this disease.
Results
Clinical findings
Age at onset of cherubism in the 24patients is given in Table 1. Of those,14 patients unveiled a positive familyhistory of cherubism (Fig. 1). The pene-

352 Meng et al.
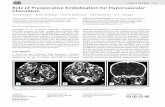
Fig. 2. Facial view shows massive enlarge-ment of the mandible.
Fig. 4. Panoramic radiographic shows bilateral lesion characterized by multilocular radiolu-cencies in the body and rami of the mandible.
trance is 66.7% (14/21) in males comparedto 48% (12/25) in females. The symptomsand signs are painless swellings of the jawin most patients (n = 20). Plump face wasapparent in 19 patients (Fig. 2), and thesclera below the pupils became exposed inonly three severe cases, giving the classic‘eye-to-heaven’ appearance. Two patientswere only mandibular protrusion.
The jaw lesions were firm and non-tender on palpation with swelling of thealveolar ridges (Fig. 3). Ten patients ran-ging in age from 9 to 20 years had painlessenlarged submandibular lymph nodes. No
Fig. 3. Intraoral view shows swelling of the alv
clinically detectable features were foundin four patients.
Radiographic findings
Nineteen patients had only mandibleinvolvement, of those, 14 were in thebilateral angles of the mandible, ascendingrami and also a considerable part of thebody of the mandible; five were in theentire mandible except for the condyles.Four patients were affected in the maxillaeas well, of those, one was in infraorbitalregion and the other three were in themaxillary tuberosities. Massive lesion inboth jaws involving the condyles may beseen in one patient.
eolar ridges.
Multilocular radiolucencies in thelesion areas were the typical feature withwell-defined edges or sclerotic borders(Fig. 4). New bone formation in the radi-olucent areas was shown in patients intheir 20s. Sclerotic thickening but noexpansion of bony cortex was observedin patients over 30 years of age (Fig. 5).The dental state in the lesion, such as dis-placement (n = 24), uneruption (n = 8),missing teeth (n = 12), or root resorption(n = 6), was observed.
Biochemical findings
Three of these patients (9-, 10-, and 12-year-old boys) showed abnormal serumphosphorus values, 1.58, 1.76, and1.83 mmol/L, respectively (normal range0.81–1.46 mmol/L), as well as abnormalalkaline phosphatase values, 435 and492 U/L, respectively, for the 9- and 12-year-old boys (normal range 20–390 U/L).However, radiographic examination of theremaining skeleton showed no furtherinvolvement of other parts of the skeletonin these three boys.
Histopathological findings
In cherubism, normal bone is partlyreplaced by pathologic tissue. Under themicroscope, it contains numerous ran-domly distributed multinucleated giantcells and vascular spaces within a fibrousconnective tissue stroma. An increase inosteoid and newly formed bone matrixwas found in the peripheral region ofthe fibrotic stroma in patients above theage of 20 years (Fig. 6). An eosinophilicperivascular cuffing was seen in 10 of the20 patients who had this examination per-formed (Fig. 7). The multinucleated giant

Clinicopathologic study 353
Fig. 5. Panoramic radiograph shows multilocular rarefactions replaced by irregular patchysclerosis, giving a ground-glass appearance.
cells were positive for tartrate resistantacid phosphatase (Fig. 8) and expressedthe vitronectin receptor.
Surgical interventions
Surgical interventions were performed in19 patients and the results after follow-upwere available for 11 patients (Table 2).Of these, 10 surgical procedures provokedno active growth of the lesional tissue. Theremaining case, a 26-year-old femalepatient with cherubism Grade IV (i.e.,an extensive cherubic lesion in both jawsinvolving the mandibular condyles) hadexperienced swelling of the jaws for 17years. Surgical intervention (the type was
Fig. 6. Multinucleated giant cells are scatteredformed bone matrix are visible (hematoxylin an
unknown) had been performed eight timesin other hospitals, but failed to arrestactive growth of remnant cherubic lesions.When the patient was referred to ourhospital, partial resection and osteoplastywas performed in the mandible, the fol-lowing year in the maxillae, and osteo-plasty in the mandiblar angle region thethird year. All surgical treatments weresuccessful without any complications aftersurgery or at later follow-ups.
Discussion
Cherubism is a rare benign bone diseasewith autosomal dominant inheritance. Itappears to have 100% penetrance in males
in vascular fibrous stroma. Osteoid and newlyd eosin stain, original magnification, �20).
but only 50–70% penetrance in females17.However the penetrance is lower in thisseries (male, 66.7%; female, 48%) and 10of the present patients are nonfamilial ashas been reported in the literature7,8. Arethese true sporadic cases of cherubism oronly apparently sporadic due to some denovo genetic mutations, incomplete pene-trance, or an inadequate family history?This question is worthy of furtherresearch.
The symptoms and signs of cherubismdepend on the severity of the condition,and range from no clinically or radiologi-cally detectable features to grotesquelydeforming mandibular and maxillaryovergrowth with respiratory embarrass-ment and impaired vision and hearing.The patients in this study had either sym-metrical swellings in the mandible or man-dibular protrusion only, and in threesevere cases, exhibited the ‘eye-to-hea-ven’ appearance described by JONES
9 withdiffuse maxillary involvement. While theswelling may be unilateral in somecases20.
Widening of the alveolar ridges is com-mon. With maxillary involvement, thealveolar widening may result in a narrowV-shaped palate, which sometimes causesbackward displacement of the tongue. Invery severe cases, the palatal vault may beobliterated, causing dysarthria, dysphagiaand dyspnoea. However, this was notobserved in any of the 24 patients in thisstudy. Intraoral examination also revealedmalocclusive and abnormal dentition,worse in the mandible. Abnormal denti-tion takes the form of premature loss ofdeciduous teeth and widely spaced, dis-placed, unerupted, or absent permanentteeth.
Painless enlargement of the submandib-ular lymph nodes frequently occurred inchildren with cherubism11. This wasobserved in 10 of our patients. It is prob-ably a reactive hyperplasia and fibrosiswhich has been confirmed by microscopicexamination of lymph node biopsy speci-mens from cherubic patients30.
Cherubism is usually an isolated diseasein an otherwise mentally and physicallynormal child. A few case reports indicatethat cherubism might be associated withother genetic disorders, such as Noonan-like syndrome or Noonan-like/multiplegiant cell lesion syndrome (MIM163955), a lesion in the humerus, gingivalfibromatosis4, and Ramon syndrome(MIM 266270)18. Cherubism has alsobeen described in an individual with fra-gile-X mental retardation16 but thisrequires further research. The diseasemay occasionally be associated with other

354 Meng et al.
Fig. 7. Multinucleated giant cells are scattered around blood vessels and eosinophilic collagenperivascular cuffing is visible (hematoxylin and eosin stain; original magnification, �100).
cystic foci, e.g., in the metacarpal bones,carpal bones, humerus, ribs, pelvis, femurand tibia4,13,32, however, in this series, nocase of cherubism involved other parts ofthe skeleton.
The biochemical markers such as serumcalcium, serum phosphorus, alkalinephosphatase and parathyroid hormoneare usually within the normal ranges withrespect to age, and therefore they mightserve to differentiate cherubism fromhyperparathyroidism, because in the latter,
Fig. 8. Multinucleated giant cells in cherubismphatase (TRAP stain; original magnification, �1
they are all elevated. However, elevatedserum phosphorus, and alkaline phospha-tase levels were found in three of thepresent cases. However, this is expectedin persons of young age. KAUGARS et al.12
postulated that the abnormalities might bedue to the patient’s difficulty in consuminga normal diet.
Radiologically, cherubism is character-ized by the quite specific finding of well-defined multilocular areas of diminisheddensity, often very extensive, with a few
are positive for tartrate resistant acid phos-00).
irregular bony septa. In adults, the multi-locular rarefactions are replaced by irre-gular patchy sclerosis, with progressivecalcification. There is a classical (butnon-specific) ground-glass appearance,as a result of the small tightly compressedtrabecular pattern.
SEWARD & HANKEY23 suggested a grad-
ing system for cherubism, based on theradiographic location of the lesions in thejaws, which has been used in later studiesand has been supplemented with severalsubdivisions in other studies1,15,19. RAMON
& ENGELBERG19 classified a case of cher-
ubism with monstrous deformity of thejaws not restricted to elevation of theorbital floor but penetrating the orbit, asseverity Grade IV. The same grade wasalso used by AYOUB & EL-MOFTY
1 in theircase of massive extension involving themandibular condyles. In our study, invol-vement of the entire mandible except thecondyles was also classified as Grade II,and both jaws involving the condyles asGrade IV, as mentioned in the ‘‘Materialsand methods’’ section of this article. Allthe classifications above are based on theextent at the time of examination. Thegrade often increases on follow-up exam-ination. MOTAMEDI
15 proposed a new grad-ing system that addresses both theinvolvement and aggression of the diseasebut it has not been widely accepted.
The clinical and radiologic criteria sug-gested by CHUONG et al.2 for classifyingcentral giant cell granulomas into aggres-sive and nonaggressive types, are alsovalid for cherubism. It is clinically justi-fied by the additional phase of rapidgrowth, the huge deformity of the man-dibular body, and especially the bilateralposterolaterosuperior extension of maxil-lary lesions, with penetration into the ret-robulbar areas of orbital cavities.Radiographically, it is justified by theperforation and disruption of wide areasof the bony cortex and marked root resorp-tion, which has long been considered to bea more aggressive process1,5,15,19,28 as wasfound in some cases presented in ourcurrent report. SILVA et al.24 reported anextreme case of cherubism that behaved ina locally aggressive manner. An 8-year-old boy presented with severe facial swel-ling. The lesion progressed rapidly and 17months later the patient died of gastroin-testinal and pulmonary infections as aresult of aspiration due to the gross defor-mity of the child.
Histological appearance of the lesionsin cherubism which is similar to that ofcentral giant cell granuloma, hyperpar-athyroidism and giant cell tumor in chil-dren and young adults, is not diagnostic.

Clinicopathologic study 355
Table 2. Types of surgical interventions and results after follow-up in 14 patients with cherubism
Types of surgical intervention Lesion area Grade Age (years) Follow-up Recurrence (Y/N)
Curettage Bilateral mandibular ascendingrami and molar regions
I 7 1 year and 2 months N
Curettage Bilateral mandibular ascendingrami and molar regions
I 9 2 years and 3 months N
Curettage Bilateral mandibular ascendingrami and molar regions
I 9 10 years and 9 months N
Curettage Bilateral mandibular ascendingrami and molar regions
I 11 15 years and 4 months N
Curettage + partial resection Bilateral mandibular ascendingrami and molar regions
I 10 5 months N
Partial resection + bone graft Entire mandible except the condyles II 15 2 years and 8 months NPartial resection + bone graft Bilateral mandibular body I 12 4 years and 1 months NOsteoplasty Entire mandible except the condyles II 20 8 months NPartial resection + osteoplasty Both jaws involving the condyles IV 28 8 months NIncisional biopsy only Bilateral mandibular ascending
rami and molar regionsI 9 3 years and 5 months Slowing growth
Incisional biopsy only Bilateral mandibular angle regions I 7 11 years QuiescenceNone Entire mandible except the condyles II 20 22 years QuiescenceNone Bilateral mandibular ascending
rami and molar regionsI 34 3 years and 5 months Quiescence
None Bilateral mandibular ascendingrami and mentis
I 2731 4 years Slowing growth
The histological profile is that of prolifer-ating vascular fibrous connective tissuewith abundant multinucleated giant cellsthat are osteoclasts since they synthesizetartrate resistant acid phosphatase, expressthe vitronectin receptor, and resorb bone.An increase in osteoid and newly formedbone matrix is found in the peripheralregion of the fibrotic stroma in the patientover 20 years of age. Eosinophilic col-lagen perivascular cuffing is reported bysome authors5,21 as a specific finding ofcherubism, but it could only be observedin 10 cases in our series. When this char-acteristic is absent, distinction from giantcell tumor and giant cell granuloma shouldbe based on clinical and radiologicalappearances.
The main biologic feature of cherubismis its natural course. This study indicatesthat the state of normalization is reachedfaster in patients of Grade I than those ofhigher grades, in which progression con-tinues but at a reduced rate until the thirddecade of life. On average, the diseasemanifests itself between the ages of 6and 10 years, with initial rapid enlarge-ment of the jaws. After puberty the lesionsbegin to regress. Jaw remodeling con-tinues through the third decade of life,at the end of which, the clinical abnorm-ality may be subtle.
The treatment of cherubism should bebased on the known natural course of thedisease and the clinical behavior of theindividual case. In some cases it resolveswithout treatment as was found in ourstudy. However, the frequency of itsoccurrence is unknown, since most of
the recorded cases have been surgicallytreated before reaching puberty.
Conservative management is appropri-ate until functional or emotional distur-bances demand surgical intervention.Curettage with or without bone graftingis the treatment of choice although it mayneed to be repeated on several occasions.We suggest curettage of the affected tis-sue, preserving the teeth as long as possi-ble. We found that curettage or surgicalcontouring during the rapid growth of thelesions not only gives good immediateresults, but also arrests active growth ofremnant cherubic lesions and even stimu-lates bone regeneration as confirmed byDUKART et al.3, although some authors22,23
reported cases in which surgery during therapid growth phase was followed by asevere relapse and a more aggressivecourse. For patients with extensive lesionsand the risk of pathologic fracture of themandible, segmental mandibulectomy fol-lowed by reconstruction with fibula freeflap is suggested. Excellent results wereobtained using this technique in twopatients in our series.
Medical therapy in the form of calcito-nin is theoretically appropriate. Calcitoninis recognized as an effective treatment forgiant cell granuloma of the jaw6, but clin-ical evidence in the literature to endorse itsapplication in cherubism is lacking. Cal-citionin has been shown to cause inhibi-tion of bone resorption by multinucleatecells in cherubic tissue in vitro26. Despiteits failure in some cases, further investiga-tion of its efficacy may be warranted,perhaps with improved compliance being
achieved by the use of intranasal ratherthan subcutaneous administration. KABAN
et al.10 reported that antiangiogenic ther-apy with daily low-dose interferon alphasuccessfully prevents the recurrence ofaggressive giant cell tumors of jaws.Under physiologic conditions interferonalpha contributes to maintenance of nor-mal bone mass by downregulating osteo-clast bone resorption27. Interferon alphaalso inhibits the production of at least oneproangiogenic protein basic fibroblastgrowth factor (bFGF), by human tumorcells25. Furthermore, KABAN et al.hypothesized that interferon may stimu-late osteoblasts and preosteoblasts andtherefore enhance bone formation. Theirreport provided a new insight for furtherstudy of therapy for cherubism.
Recent genetic advances have beenmade in relation to cherubism with theidentification of the gene to SH3BP229.All the mutations identified so far8,14,29 arelocated in exon 9 and result in amino acidsubstitutions within a 6 amino acidsequence from positions 415 to 420. Thismay represent a specific protein domainwhich, when disrupted, leads to the cher-ubism phenotype. This brings us one stepcloser to the elixir of gene therapy. How-ever, for the moment at least, managementof this condition remains in the realm ofthe specialist surgeon. We recommendthat surgical intervention not necessarilybe postponed until after puberty.
Acknowledgments. This work was sup-ported by the ‘‘National Nature ScienceFoundation’’ of China (No. 30271412).

356 Meng et al.
The authors would like to acknowledgeYun-tang Wu for his generous help inevaluation of radiographs.
References
1. Ayoub AF, El-Mofty SS. Cherubism:report of an aggressive case and review ofthe literature. J Oral Maxillofac Surg1993: 51: 702–705.
2. Chuong R, Kaban LB, Kozakewich H,Perez-Atayde A. Central giant celllesions of the jaws: a clinicalpathologicstudy. J Oral Maxillofac Surg 1986: 44:708–713.
3. Dukart RC, Kolodny SC, Polte HW,Hooker SP. Cherubism: report of case. JOral Surg 1974: 32: 782.
4. Dunlap C, Neville BM, Vickers RA,O’Neil D, Barker B. The Noonan syn-drome/cherubism association. Oral SurgOral Med Oral Pathol 1989: 67: 698–705.
5. Hammer JE. The demonstration of peri-vascular collagen deposition in cherub-ism. Oral Surg Oral Med Oral Pathol1969: 27: 129.
6. Harris M. Central giant cell granulomasof the jaws regress with calcitonin ther-apy. Br J Oral Maxillofac Surg 1993: 31:89–94.
7. Hitomi G, Nishide N, Mitsui K. Cheru-bism: diagnostic imaging and reviewof the literature in Japan. Oral SurgOral Med Oral Pathol 1996: 81: 623–628.
8. Imai Y, Kanno K, Moriya T, Kayano
S, Seino H, Matsubara Y, Yamada
AA. A missense mutation in the SH3BP2gene on chromosome 4p16.3 found in acase of nonfamilial cherubism. CleftPalate Craniofac J 2003: 40: 632–638.
9. Jones WA. Familial mutilocular cysticdisease of the jaws. Am J Cancer 1933:17: 946.
10. Kaban LB, Troulis MJ, Ebb D, August
M, Hornicek FJ, Dodson TB. Antian-giogenic therapy with interferon alpha forgiant cell lesions of the jaws. J OralMaxillofac Surg 2002: 60(10):1103–1111.
11. Katz JO, Underhill TE. Multilocularradiolucencies. Dent Clin North Am1994: 8: 63–81.
12. Kaugars GE, Niamtu III J, Svirsky JA.Cherubism: diagnosis, treatment andcomparison with central giant cell gran-ulomas and giant cell tumors. Oral SurgOral Med Oral Pathol 1992: 73: 369–374.
13. Lannon DA, Earley MJ. Cherubismand its charlatans. Br J Plastic Surg2001: 54: 708–731.
14. Lo B, Faiyaz-U1-Haque M, Kenned
YS, Aviv R, Tsui LC, Teebi AS. Novelmutation in the gene encoding c-Abl-binding protein SH3BP2 causes cheru-bism. Am J Med Genet 2003: 121A:37–40.
15. Motamedi MHK. Treatment of cherub-ism with locally aggressive behavior pre-senting in adulthood: report of four casesand a proposed new grading system. JOral Maxillofac Surg 1998: 56: 1336–1342.
16. Peters WJN. Cherubism: a study oftwenty cases from one family. Oral Surg1979: 47: 307–311.
17. Quan F, Grompe M, Jakobs P, Popo-
vich BW. Spontaneous deletion in theFMR1 gene in a patient with fragile Xsyndrome and cherubism. Hum MolGenet 1995: 4: 1681–1684.
18. Ramon Y, Berman W, Bubis JJ. Gingi-val fibromatosis associated with cherub-ism. Oral Surg Oral Med Oral Pathol1967: 24: 435–448.
19. Ramon Y, Engelberf IS. An unusuallyextensive case of cherubism. J Oral Max-illofac Surg 1986: 44: 325–328.
20. Reade PC, McKellar GM, Radden
BG. Unilateral mandibular cherubism:brief review and case report. Br J OralMaxillofac Surg 1984: 22: 189–194.
21. Regezi JA, Sciubba J. Oral Pathology,Clinical-Pathologic Correlations. 2ndedn. Philadelphia, PA: Saunders 1993.
22. Riefkohl R, Georgiade GS, Geor-
giade NG. Cherubism. Ann Plast Surg1985: 14: 85–90.
23. Seward GR, Hankey GT. Cherubism.Oral Surg Oral Med Oral Pathol 1957: 10:952.
24. Silva EC, de Souza PEA, Barreto DC,Dias RP, Gomez RS. An extreme case ofcherubism. Br J Oral Maxillofac Surg2002: 40: 45–48.
25. Singh RK, Fidler IJ. Systemic admin-istration of interferons for inhibition of
cancer metastasis. In: Stuart-Harris R,Penny R, eds: Clinical Applications ofthe Interferons. London, UK: Chapman &Hall 1997: 391–404.
26. Southgate J, Sarma U, Townend JV,Barron J, Flanagan AM. Study ofthe cell biology and biochemistry ofcherubism. J Clin Pathol 1998: 51:831–837.
27. Takayanagi H, Kim S, Matsuo Ket al. RANKL maintains bone homeo-stasis through c-Fos-dependent induc-tion of interferon-beta. Nature 2002:416: 744.
28. Timosca GC, Galesanu RM, Cotutiu
C, Grigoras M. Aggressive form of cher-ubism: report of a case. J Oral MaxillofacSurg 2000: 58: 336–344.
29. Ueki Y, Tiziani V, Santanna C, Fukai
N, Maulik C, Garfinkle J, Ninomiya
C, doAMARAI c, peters H, Habal M,Rhee-Morris L, Doss JB, Kreiborg S,Olsen BR, Reichenberger E. Muta-tions in the gene encoding c-Abl-bindingprotein SH3BP2 cause cherubism. NatGenet 2001: 28: 125–126.
30. von Wowern N. Cherubism. Int J OralSurg 1972: 1: 240–249.
31. Wang ZP, Sun DX. Cherubism, report of4 cases. Shanghai Stomatol 1995: 4: 120–121.
32. Wayman JB. Cherubism: a report onthree cases. Br J Oral Surg 1978: 16:47–56.
33. Wu YT, Sun KW, Zhu XP. Familialcherubism (reports of 10 cases). Chin JStomatol 1993: 28: 148–150.
34. Xu M, Gao CY. Cherubism 1 case report.Chin J Stomatol 1992: 27: 184.
35. Yang GM. Familial fibrous dysplasia ofbone in bilateral jaws: 1 case report. JPract Stomatol 1998: 14: 306–307.
Address:Xue-Mei MengDepartment of Oral PathologyPeking University School of Stomatology22 South Zhongguancun AvenueHaidian DistrictBeijing 100081PR China.Tel: +86 10 62179977x2201Fax: +86 10 62173402E-mail: [email protected]