Cardiac Adrenergic Signaling.
Click here to load reader
-
Upload
franciscrick69 -
Category
Documents
-
view
212 -
download
0
Transcript of Cardiac Adrenergic Signaling.

1196
·
N Engl J Med, Vol. 347, No. 15
·
October 10, 2002
·
www.nejm.org
The New England Journal of Medicine
Editorials
A
DRENERGIC
-R
ECEPTOR
P
OLYMORPHISMS
AND
H
EART
F
AILURE
ONGESTIVE heart failure is the eventual out-come of diverse myocardial insults, including
postischemic remodeling, primary cardiomyopathies,and infections.
1
Even for specific forms of myocardialinjury, it remains difficult to predict the developmentof congestive heart failure. Racial differences in the in-cidence of congestive heart failure, reduced penetrancein families with familial cardiomyopathy, and strain-specific effects in animal models all suggest that someof the variation in the general population with regardto susceptibility to heart failure is heritable.
2
Althoughearly events in the evolution of congestive heart failureare often unknown, the final common pathways areslowly being elucidated. The role of neurohormonalactivation in the progression of heart failure is well de-fined and has been validated by the salutary effects ofangiotensin-converting–enzyme inhibition and beta-blockade on morbidity and mortality in patients withcongestive heart failure.
3-5
Variation among races andamong persons in the responses to these agents hasmade polymorphism in both renin–angiotensin andadrenergic signaling a focus of current research.
6
There is substantial polymorphism, or sequencevariation, in genes within the general population. Thefunctional importance of these variants is now beingexplored. There have been numerous studies relatingparticular polymorphisms in adrenergic-receptor genesto disparate disease outcomes or to differential re-sponses to drugs.
7,8
Underlying heterogeneity restrictsthe usefulness of such studies of genetic associationsin the discovery of major causal genes, but this ap-proach may be a powerful way to identify smaller,population-wide, heritable effects.
In this issue of the
Journal,
Small et al.
9
describean association, found retrospectively, between twopolymorphisms in adrenergic-receptor genes and therisk of congestive heart failure in a black population.Subjects who were homozygous for a deletion poly-morphism in the
a
2C
-adrenergic receptor were morethan five times as likely to have heart failure as thosewith other genotypes at the same locus. There wasalso evidence of a potential synergistic interaction insubjects who were homozygous both for the dele-tion,
a
2C
Del322–325, and for a second polymorphism(Arg389) in the
b
1
-adrenergic receptor. These poly-morphisms might be predicted, on the basis of in vitrostudies, to result in markedly increased cardiac adre-nergic stimulation. These provocative data raise thepossibility that relatively common gene variants may
C
identify persons who are at increased risk for conges-tive heart failure, but the findings must be interpretedin the context of what is known of the genetics andintegrated physiology of the entire adrenergic system.
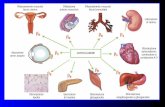
Adrenergic signaling has been studied extensively.There are multiple subtypes of receptors that trans-duce independent signals while allowing sophisticatedcross-talk and feedback regulation at many levels. Thebasic components of cardiac adrenergic signaling areshown in Figure 1.
When the heart fails, chronic sympathetic activationresults in selective down-regulation of
b
1
-adrenergicreceptors and uncoupling of both
b
1
- and
b
2
-adre-nergic receptors from G proteins, markedly blunting
b
-adrenergic–receptor signaling. Families of regulatorand adaptor molecules, which control the amplitude,the duration, and the targeting of adrenergic-recep-tor signals, are intimately involved in this blunting.There are many mechanisms for turning off signalstransduced by G-protein–coupled receptors. Mem-bers of the G-protein–coupled receptor kinase family(GRK1 through GRK6) phosphorylate adrenergic re-ceptors only when occupied by an agonist. This phos-phorylation increases the affinity of adrenergic recep-tors for cytosolic proteins, known as
b
-arrestins, whichprevent G-protein coupling. The
b
-arrestins also actas scaffolding molecules, coupling the adrenergic re-ceptors to different downstream effectors. There is alsoevidence that
b
-adrenergic receptors are involved inthe regulation of both survival of and apoptotic sig-naling in cardiomyocytes.
a
-Adrenergic signaling also has an important rolein congestive heart failure.
a
1
-Adrenergic receptors,when stimulated, are potent mediators of cardiomy-ocyte hypertrophy, initially representing a response toincreased workload but thought ultimately to be mal-adaptive. On the other hand,
a
2
-adrenergic receptors(specifically the
a
2A
- and
a
2C
-adrenergic receptors) op-erate as presynaptic inhibitory receptors that controlthe release of norepinephrine.
10
Although
b
1
-adrener-gic–receptor antagonists have negative short-term ef-fects on chronotropy and inotropy, the use of selective
b
1
-adrenergic–receptor antagonists improves ventric-ular function and survival in patients with moderateor severe congestive heart failure.
With multiple receptors and regulatory pathwaysoperating in different cells and tissues, often in coun-terbalancing directions within a hierarchical regulatorynetwork, it is difficult to predict the net outcome ofany single perturbation without whole-animal models.Fortunately, genetic models offer insight into the in-tegrated physiology of the adrenergic system and itseffects on heart failure. Transgenic-overexpression andknockout models have been made for many of the re-ceptors, and these animals are now being used to ex-plore the effects of modulating adrenergic signaling in

EDITORIALS
N Engl J Med, Vol. 347, No. 15
·
October 10, 2002
·
www.nejm.org
·
1197
a2C
a2A
b1b2
b3
Cardiac-cellmembrane
Norepinephrine
Sympatheticnerve
Gs Gi
Gi
Gs
Gs
Adenylatecyclase
Contractility Hypertrophy,heart failure
Mitogen-activatedprotein kinase
Nosynthase
+ + + +–
Figure 1.
The Basic Components of Cardiac Adrenergic Signaling.The adrenergic receptors are G-protein–coupled transmembrane receptors. On binding to ligands, the G proteins dissociate from theintracellular domain of the receptor to propagate signals by modulating the activity of downstream effector molecules such as aden-ylate cyclase, phospholipases, and ion channels. The
b
1
-Adrenergic receptor and
b
2
-adrenergic receptor are both normally coupled tothe stimulatory G protein (G
s
), and through this protein, they activate adenylate cyclase, augmenting myocyte contractility and induc-ing myocyte hypertrophy or heart failure. This regulation of cell growth involves multiple downstream molecules including mitogen-activated protein kinases in a complex regulatory network.
b
2
-Adrenergic receptors may also couple to the inhibitory G protein (G
i
)and reduce adenylate cyclase activity while activating the mitogen-activated protein kinase pathway. The role of the
b
3
-adrenergic re-ceptors is less clear, but they seem to activate nitric oxide synthase through G
i
.
a
-Adrenergic signaling also has an important role incardiac physiology. When stimulated,
a
1
-adrenergic receptors (not shown) are potent mediators of cardiomyocyte hypertrophy, actingthrough another subtype of G protein (G
q
), which stimulates mitogen-activated protein kinase pathways. The two subtypes of
a
2
-adre-nergic receptors (
a
2A
and
a
2C
) depicted here operate as presynaptic inhibitors of norepinephrine release, but are also known to havepostsynaptic functions. The
a
2A
-adrenergic receptor is important for central and peripheral nervous system inhibition of norepineph-rine release at high action-potential frequencies, whereas the
a
2C
-adrenergic receptor is more important for regulating lower-frequencystimulation. These
a
2
-adrenergic receptors inhibit the release not only of norepinephrine but also of other neurotransmitters that are notligands for the adrenergic receptors. (Courtesy of Drs. Stefan Engelhardt and Lutz Hein, University of Würzburg, Würzburg, Germany.)

1198
·
N Engl J Med, Vol. 347, No. 15
·
October 10, 2002
·
www.nejm.org
The New England Journal of Medicine
a range of mouse models of heart failure.
11,12
Althoughsuch models may not reflect perfectly the pathogenesisof human congestive heart failure, they confirm thatthe effects of individual genetic differences must beintegrated carefully into whole-animal responses topathologic stimuli.
How, then, should we interpret the findings ofSmall et al.? Given the available data, the effects of thepolymorphisms are biologically plausible. Both the
b
1
Arg389 variant and the
a
2C
Del322–325 polymor-phism have important effects on receptor function invitro. Cardiac overexpression of the
b
1
-adrenergic re-ceptor in mouse models results in severe congestiveheart failure,
11
which is consistent with the findings inthe patients studied by Small et al. Furthermore, micethat are deficient in
a
2C
-adrenergic receptors, whensubjected to aortic banding, have a markedly higherincidence of progression to heart failure and deaththan wild-type littermates
10
— another finding thatsupports the described associations. Future modelingof underactivity of the
a
2C
-adrenergic receptor andglobal overactivity of the
b
1
-adrenergic receptor willhelp to confirm potential synergy and will clarifywhether the resultant sustained sympathetic stimula-tion alone is sufficient to cause congestive heart fail-ure or whether there is also a depressed reserve in-volved in causing myocardial injury.
Another important question originating from thisstudy is whether the synergistic polymorphisms canexplain the higher morbidity and mortality from heartfailure among black patients and their differential re-sponses to drugs. Symptoms of heart failure developat an earlier age in blacks, and they are more likelythan nonblacks to have associated hypertension, di-abetes, and renal failure.
2
Furthermore, blacks withheart failure obtain less benefit than nonblacks fromthe use of angiotensin-converting–enzyme inhibitorsand beta-blockers.
6,13
Could these differences betweenblacks and nonblacks be partially explained by thepolymorphisms considered in this study?
Despite the plausibility of the results, there areimportant limitations to genetic-association studies
14
such as those described by Small et al. The limitationsinclude the possibility of genetic stratification of thepopulation resulting in a spurious association betweena polymorphism and a disease simply because boththe disease and the unlinked sequence variant arefound in the same subpopulation. It is difficult to at-tribute causality to a particular polymorphism, sinceeach polymorphism is inherited together with manyother variants, either elsewhere in the candidate geneor in other nearby genes on the same chromosome,in so-called linkage disequilibrium. For example, bothloci considered in the study by Small et al. are in ge-nomic segments close to genes encoding other ad-renergic-signaling molecules — the
a
2A
-adrenergic
receptor and
GRK5
genes, in the case of the
b
1
-adre-nergic receptor, and the
GRK4
gene, in the case of the
a
2C
-adrenergic receptor. Functionally important se-quence variants in these other genes may be in linkagedisequilibrium with the polymorphisms described,thus confounding the results. Prospective replicationof these important findings in genetically distinct pop-ulations, with the use of extended haplotypes to en-compass neighboring genes, will be a critical confirm-atory step.
15
Studies in much larger populations ofblack patients with congestive heart failure will be re-quired. It will be interesting to see whether these poly-morphisms act as modifier loci in monogenic forms ofheart failure. As the genome projects unravel the ex-tent of common genetic variation, it may be possibleto define the role of such variation in disease only withthe use of models that permit the study of both mul-tiple genetic manipulations and integrated physiology.
R
OGER
J. H
AJJAR
, M.D.C
ALUM
A. M
AC
R
AE
, M.B., C
H
.B.
Massachusetts General HospitalBoston, MA 02114
REFERENCES
1.
Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med 1994;331:1564-75.
2.
Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med 1999;340:609-16. [Erratum, N Engl J Med 1999;341:298.]
3.
Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-7.
4.
Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996;334:1349-55.
5.
The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13.
6.
Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to an-giotensin-converting–enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med 2001;344:1351-7.
7.
Wagoner LE, Craft LL, Singh B, et al. Polymorphisms of the beta(2)-adrenergic receptor determine exercise capacity in patients with heart fail-ure. Circ Res 2000;86:834-40.
8.
Liggett SB, Wagoner LE, Craft LL, et al. The Ile164 beta2-adrenergic receptor polymorphism adversely affects the outcome of congestive heart failure. J Clin Invest 1998;102:1534-9.
9.
Small KM, Wagoner LE, Levin AM, Kardia SLR, Liggett SB. Synergis-tic polymorphisms of
b
1- and
a
2C
-adrenergic receptors and the risk of con-gestive heart failure. N Engl J Med 2002;347:1135-42.
10.
Brede M, Wiesmann F, Jahns R, et al. Feedback inhibition of catechol-amine release by two different a2-adrenoreceptor subtypes prevents pro-gression of heart failure. Circulation (in press).
11.
Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertro-phy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A 1999;96:7059-64.
12.
Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-span-ning receptors and heart function. Nature 2002;415:206-12.
13.
The Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659-67.
14.
Cardon LR, Bell JI. Association study designs for complex diseases. Nat Rev Genet 2001;2:91-9.
15.
Current human genome assembly at National Center for Biotechnol-

EDITORIALS
N Engl J Med, Vol. 347, No. 15
·
October 10, 2002
·
www.nejm.org
·
1199
ogy Information:
Homo sapiens
genome view. Bethesda, Md.: National Center for Biotechnology Information, 2002. (Accessed September 20, 2002, at http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/map_search?chr=hum_chr.inf&query.)
Copyright © 2002 Massachusetts Medical Society.
A
NTIMICROBIAL PEPTIDES IN HEALTH AND DISEASE
ICROBES surround us. However, most of thetime we are unaware of them. Our epithelial sur-
faces, which provide the physical barrier separating usfrom the environment, are usually free of signs of abattle taking place. Over the past several years, wehave come to realize that the epithelium is capable ofmounting its own battery of defensive chemicals,which are more extensive, more potent, and more ex-quisitely regulated than initially appreciated. I am re-ferring not to the constituents of fluids, such as tears,saliva, and sweat, that are secreted onto the surface ofepithelia, but to antimicrobial peptides, the newly de-scribed components that defend this barrier.1 Thesepeptides can mount a defense that is as powerful asthat provided by any of the body’s circulating cellsthat are traditionally associated with protection.
Much has been learned from the immunity of in-sects, in part because it was clear from the start thattheir defenses could not depend on antibodies or lym-phocytes, since they lack all the equipment of adaptiveimmunity. Antimicrobial peptides protect mucosal anddry epithelial surfaces of all multicellular organisms.1
These molecules are widely distributed in nature. Theyare very diverse with respect to amino acid sequenceand secondary structure but share certain properties,such as an affinity for the negatively charged phospho-lipids that are present on the outer surfaces of the cy-toplasmic membranes of many microbial species. Morethan 500 different antimicrobial peptides have beendiscovered in organisms from insects to humans. Theyare encoded by genes, synthesized as precursors, andprocessed by specific proteases into mature, activeforms either before or after secretion. They target themicrobial membrane, acting like disinfectants and kill-ing rapidly. Most have a broad spectrum of activity,including activity against bacteria, fungi, viruses, andfrequently transformed cells. Most organisms synthe-size several different types of peptide within their ep-ithelial surfaces, creating a broad-spectrum antimicro-bial cocktail designed to defend the epithelium fromthe organisms most likely to mount an attack. Becauseit is difficult for a microbe to change the phospholipidorganization of its membrane, resistance to the pep-tides occurs at levels that are orders of magnitude low-er than those observed for conventional antibiotics.
M
Indeed, it is likely that these antimicrobial weaponshave been fundamental to the evolution of multicel-lular organisms within the microbial environment.
Two major classes of antimicrobial peptides in mam-mals have been described: defensins and cathelicidins.1
The defensins themselves are divided into two classes,a and b, on the basis of the differences in their sec-ondary structures. a-Defensins were first discoveredin the granules of white cells. Several years later, theywere found in Paneth cells, the granule-containing cellsthat lie in the base of the crypts of the small intestine.In white cells, a-defensins contribute to nonoxidativekilling. In Paneth cells, they are secreted into the cryptsand control the growth of bacteria in the small bow-el. In humans, b-defensins are synthesized by all ep-ithelial tissues.1,2 Four human b-defensins have beenidentified (although more than 20 genes appear to ex-ist in our genome3). Human b-defensin 1 (HBD-1) isgenerally produced constitutively, whereas the othersare inducible. Cathelicidins are a large and ancientclass, traced from hagfish through humans.4 They aresecreted in neutrophils and can be found in the blood-stream after infection. On epithelial surfaces, theyfunction like defensins.
Defensins and cathelicidins perform several func-tions. They preserve epithelia by remaining associatedwith tissues, as in the case of the skin, lips, tongue, andrectum. In other settings, such as the moist airways,gastrointestinal tract, and urinary tract, they are alsosecreted into the biofilm covering the epithelial sur-face, where they create a barrier that is chemically le-thal to microbes. Certain antimicrobial peptides pro-mote epithelial growth and angiogenesis.4 Defensinsand cathelicidins are also chemokines, substances thatattract migrating or circulating cells.5,6 Defensins canattract immature dendritic cells and T lymphocytesto sites of injury or infection; the LL-37 cathelicidinacts on a specific G protein–coupled receptor to at-tract neutrophils.6
The receptors that recognize the presence of mi-crobes, damaged tissues, or proinflammatory cytokinesand subsequently induce the expression of antimicro-bial-peptide genes have recently been described. Thebest characterized are the Toll-like receptors (TLRs),of which the interleukin-1 receptor is a prototype.7
These receptors are displayed on epithelial surfaces andon circulating cells that are regarded as the front line,such as dendritic cells and macrophages. At least 10TLRs have been identified in humans, and each ap-pears to recognize a distinct array of microbial con-stituents, representing pathogen-associated microbi-al patterns. Banks of antimicrobial-peptide genes areprobably hard-wired, through intracellular circuitslinked to batteries of specific TLRs, to respond to or-ganisms with which we have evolved. Sustained activityof this system can also cause disease.8