BIOMECHANICS OF TRABECULAR B - Union Collegeorzo.union.edu/~curreyj/BNG-345_files/Keaveny et al...
Transcript of BIOMECHANICS OF TRABECULAR B - Union Collegeorzo.union.edu/~curreyj/BNG-345_files/Keaveny et al...
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
Annu. Rev. Biomed. Eng. 2001. 3:307–33Copyright c© 2001 by Annual Reviews. All rights reserved
BIOMECHANICS OF TRABECULAR BONE
Tony M. Keaveny1,2, Elise F. Morgan1, Glen L. Niebur3,and Oscar C. Yeh11Orthopaedic Biomechanics Laboratory, Department of Mechanical Engineering,University of California, Berkeley, California 94720-1740,2Department of OrthopaedicSurgery, University of California, San Francisco, California 94143 and3Department ofAerospace and Mechanical Engineering, University of Notre Dame, Notre Dame, Indiana46556; e-mail: [email protected]; emorgan@biomech3. me.berkeley.edu;[email protected]; [email protected]
■ Abstract Trabecular bone is a complex material with substantial heterogeneity.Its elastic and strength properties vary widely across anatomic sites, and with agingand disease. Although these properties depend very much on density, the role of archi-tecture and tissue material properties remain uncertain. It is interesting that the strainsat which the bone fails are almost independent of density. Current work addresses theunderlying structure-function relations for such behavior, as well as more complexmechanical behavior, such as multiaxial loading, time-dependent failure, and damageaccumulation. A unique tool for studying such behavior is the microstructural classof finite element models, particularly the “high-resolution” models. It is expected thatwith continued progress in this field, substantial insight will be gained into such im-portant problems as osteoporosis, bone fracture, bone remodeling, and design/analysisof bone-implant systems. This article reviews the state of the art in trabecular bonebiomechanics, focusing on the mechanical aspects, and attempts to identify importantareas of current and future research.
CONTENTS
INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 308TRABECULAR BONE COMPOSITION AND MICROSTRUCTURE. . . . . . . . . . . 308HETEROGENEITY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 309ANISOTROPY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 310RELATIVE ROLES OF DENSITY (VOLUME FRACTION),ARCHITECTURE, AND TISSUE MATERIAL PROPERTIESON TRABECULAR ELASTIC BEHAVIOR . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 314
STRENGTH . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 316POSTYIELD AND DAMAGE BEHAVIOR . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 319
1523-9829/01/0825-0307$14.00 307
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
308 KEAVENY ET AL
CREEP AND FATIGUE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 321MICROMECHANICAL FINITE ELEMENT MODELING . . . . . . . . . . . . . . . . . . . . 322CONCLUDING REMARKS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 325
INTRODUCTION
Research on the biomechanics of trabecular bone has been ongoing for over 30years and is still intensely active. Motivated mostly by the need to understand therole of trabecular bone in age-related bone fracture and the design of bone-implantsystems, this work has addressed characterization of mechanical properties as afunction of such variables as anatomic site, density, and age. A number of reviewshave summarized that work (1–3). As sophisticated engineering analysis toolsrequiring more precise input data for optimal performance, such as bone-specificfinite element modeling (4–14), have been developed, emphasis is now turning toa more complete characterization of the mechanical properties. In addition, thereis growing interest in the role of trabecular bone damage in both weakening wholebones and stimulating biological remodeling. The focus of this review is to presentan overview of this latest body of work with an emphasis on the mechanical aspectsand to indicate important areas of future research.
TRABECULAR BONE COMPOSITIONAND MICROSTRUCTURE
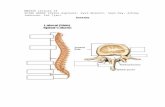
Trabecular bone is the spongy, porous type of bone found at the ends of all longbones and found within flat and irregular bones, such as the sternum, pelvis, andspine (Figure 1). The microstructural struts or trabeculae that make up a speci-men of trabecular bone are composed of trabecular tissue material. The trabeculaeenclose a three-dimensional, interconnected, open porous space, resulting in acellular solid (15) type of material. The pores are filled with bone marrow andcells in vivo. The scale of these pores is on the order of 1 mm, and the scaleof the trabecular thickness is an order of magnitude lower. We are concernedmostly with the behavior of small specimens of trabecular bone, on the orderof 5–10 mm in dimension, a scale at which the bone behaves as a continuum(16, 17). The trabecular microstructure is typically oriented, such that there isa “grain” direction along which mechanical stiffness and strength are greatest.This microstructural directionality gives trabecular bone anisotropy of mechani-cal properties. The trabecular tissue material itself is morphologically similar tocortical bone (an anisotropic composite of hydroxyapatite, collagen, water, andtrace amounts of other proteins) but is arranged in “packets” of lamellar bone (18).Thus, trabecular bone is classified from an engineering materials perspective as acomposite, anisotropic, open porous cellular solid. Like many biological materi-als, it displays time-dependent behavior, as well as damage susceptibility duringcyclic loading.
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 309
Figure 1 Volume rendering (20-µm resolution) of (a) bovine proximal tibial, (b) hu-man proximal tibial, (c) human femoral neck, and (d ) human vertebral trabecular bone.All specimens have the same bulk dimensions (3× 3 × 1 mm3).
HETEROGENEITY
A critical issue that distinguishes trabecular bone from many other biological tis-sues is its substantial heterogeneity, which leads to wide variations in mechanicalproperties1. This heterogeneity results from underlying variations in volume frac-tion, “architecture” (i.e. the three-dimensional arrangement of the individual tra-beculae), and tissue properties, in that order of importance. For example, com-pressive modulus can vary 100-fold from one location to another within a singleproximal tibia (19) (Figure 2), and strength can vary fivefold within the proximalfemur (20). Across sites and species, mean values of modulus and strength candiffer by more than an order of magnitude (Table 1). Substantial loss of mechanical
1Because trabecular bone spans multiple length scales, it is important to distinguish betweenmechanical behavior at the level of the whole specimen—the apparent properties—as op-posed to that at the level of individual trabeculae—the tissue properties. Thus, for example,we talk of apparent versus tissue modulus for trabecular bone for the whole specimen andtrabecular tissue, respectively. Unless noted otherwise, we refer to material properties atthe apparent level.
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
310 KEAVENY ET AL
Figure 2 Elastic moduli from a proximal transverse section of a human tibia. Circledvalues are measured moduli (in megapascals) of specimens taken from that region.(From Reference 19, with permission.)
properties also occurs with aging in humans. For example, ultimate stress is reducedby almost 7% and 11% per decade for the human proximal femur and spine, re-spectively, from ages 20–100 (21–23) (Figure 3). Strength does not decrease inany significant manner until after about age 30, perhaps even later depending onsite (22–24).
Because of the substantial heterogeneity of trabecular bone, factors such asage and site need to be designated when discussing the specifics of the mechanicalproperties, i.e. trabecular bone from the aged spine is much different than that fromthe young hip. This is a key concept in trabecular bone biomechanics and has directrelevance to such fields as tissue engineering, where the goal is to replace damagedtrabecular bone with a substitute having appropriate mechanical properties for thatsite.
ANISOTROPY
Trabecular bone is anisotropic in both modulus (22, 25–27) and strength (22, 28).The extent of anisotropy is mild compared with such materials as fiber-reinforcedcomposites, but its biomechanical significance in terms of whole bone strength
TABLE 1 Mean values of modulus and ultimate strength for various anatomic sites
Modulus Ultimate StrengthAnatomic site (reference) Age Range (MPa) (MPa)
Vertebra (22) 15–87 67± 45 2.4± 1.6
Proximal tibia (63) 59–82 445± 257 5.3± 2.9
Proximal femur (132) 58–85 441± 271 6.8± 4.8
Calcaneus (48) Not reported 68± 84 1.4± 1.3
Bovine proximal tibia (71) Not applicable 2380± 777 24± 8.3
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 311
Figure 3 Dependence of compressive strength on age for human vertebral and prox-imal femoral trabecular bone cores. (Adapted from References 21 and 23, with per-mission.)
or bone-implant performance remains to be quantified. We know, for example,that as trabecular bone becomes more porous, its compressive strength becomesmore anisotropic (Figure 4), but the significance of this in hip fracture etiologyis not known. Consistent with the concepts set forth in Wolff’s law (29–31), itappears that the anisotropy develops as a form of adaptive response to functionalloading—bone is placed where it most needs to be. Trabecular bone possessesat least orthotropic symmetry (17, 32), in some instances displaying transverseisotropy (33). As an example of the degree of trabecular anisotropy, mean values ofstrength and modulus of human vertebral bone in the superior-inferior direction arehigher than those in the transverse direction by factors of 2.8 and 3.4, respectively(22). For a single specimen of bovine femoral bone, ratios of maximum modulusto that in an orthogonal direction can be as high as 7.4 (34).
Mechanical testing of a specimen of trabecular bone in its principal materialcoordinate system—which is required for measurement of the intrinsic anisotropicmaterial behavior—requires knowledge of the orientation of this coordinate sys-tem a priori. Strong evidence now exists that the principal material directions oftrabecular bone are aligned with the principal structural directions of the trabe-cular architecture (17, 33). Thus, mechanical testing should be performed alongthe principal directions of the trabecular architecture. If this is not done, the result-ing measurements are difficult to interpret because they will be influenced by suchmultiple material constants as elastic modulus and Poisson’s ratio in the main andtransverse directions, as well as by shear moduli (35).
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
312 KEAVENY ET AL
Fig
ure
4Y
ield
stre
ssfo
rlon
gitu
dina
lver
sus
tran
sver
selo
adin
g,an
dth
est
reng
than
isot
ropy
ratio
(SA
R),
plot
ted
asa
func
tion
ofap
pare
ntde
nsity
for
thre
edi
ffer
entl
oadi
ngm
odes
(com
pres
sion
,ten
sion
,and
shea
r).(
From
Ref
eren
ce80
,with
perm
issi
on.)
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 313
Figure 5 A representation of the anisotropic nature of trabecular bone architecture(left) and the measured fabric ellipse (right). The principal radii of the fabric ellipseare denoted bya andb. θ is the angle between the measurement coordinate system andthe principal fabric directions. (From Reference 44, with permission.)
Efforts to quantify the principal structural directions and the structural anisotropyof trabecular bone go back at least 25 years. In the early 1970s, Whitehouse (36, 37)analyzed histological sections of trabecular bone using measurements of meanintercept length (MIL), the mean length of a line segment passing through thespecimen that lies entirely within bone tissue. He found that a polar plot of MILversus the angle of the line segment can be fit to an ellipse (Figure 5). The majorand minor axes of the ellipse quantify the structural anisotropy of the specimen.In the early 1980s, Harrigan & Mann (38) observed that the MIL measurements inthree dimensions are equivalent to a positive definite second-order tensor definingan ellipsoid.
Cowin (39) classified the inverse of the MIL tensor as a type of fabric tensor, ameasure of local structural anisotropy. He developed a general theory relating thefabric tensor to the anisotropic elastic behavior of a material. Based on this theory,the ratios of the eigenvalues of the fabric tensor quantify the degree of structuralanisotropy. The specimen is orthotropic if there are three distinct eigenvalues,transversely isotropic if there are two repeated eigenvalues, and isotropic if theyare all equal. This theory was then applied to trabecular bone, using the inversesquare root of the MIL ellipsoid as the fabric tensor (31). The 1990s saw specificapplications of this theory to trabecular bone (34, 40, 41), as well as other empiricalstudies relating fabric-derived measures of structural anisotropy to mechanicalproperties (17, 25, 42–44).
By the mid-1990s, the fabric tensor had become, and still is, the standard quan-titative descriptor of trabecular orientation and structural anisotropy. Indeed, it isnow routine to construct this ellipsoid from a three-dimensional image of a spec-imen derived from sophisticated imaging techniques, such as micro-computedtomography (45–47), micro-magnetic resonance imaging (48, 49), or automatedserial sectioning (50, 51). Other tensorial fabric measures have been developedand applied to trabecular bone, most notably volume orientation, star volume
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
314 KEAVENY ET AL
distribution, and star length distribution methods (52–54). This collective researchhas been driven by the need to better understand the role of trabecular architecturein loss of mechanical properties with aging and osteoporosis, an important issuediscussed in more detail below.
RELATIVE ROLES OF DENSITY (VOLUME FRACTION),ARCHITECTURE, AND TISSUE MATERIAL PROPERTIESON TRABECULAR ELASTIC BEHAVIOR
The elastic modulus (and failure stress) of trabecular bone depends primarily onapparent density (the product of volume fraction and trabecular tissue density,the latter being essentially constant at about 2 g/cm3). However, the precise formof this relationship remains controversial. In retrospect, it appears that most ofthe controversy stems from the dependence of this relationship on anatomic site(55) and loading direction (39, 56), and from the imprecision introduced by ig-noring anisotropy and end-artifact effects (57–64) in the mechanical tests. Endartifacts arise from damage incurred at the ends of machined specimens whenthey are tested in compression between platens with no other means of attach-ment to the load frame. If strains are computed from the relative displacement ofthe platens, substantial systematic underestimation and random errors can occur(59). The known dependence of the modulus-density relationship on loading di-rection (39, 56) implies that experimental protocols must either control or correctfor specimen anisotropy. Although cubic (65) and squared (66) modulus-densityrelationships have been reported for multiple sites pooled together, the relation-ship appears to be linear for on-axis loading (loading along the main trabecularorientation) within a single site (28, 55, 67) (Figure 6). The limited range in den-sity found within a single anatomic site renders the differences in the predictedmodulus values between linear and power law relationships negligible.
A full understanding of the modulus-density relation requires an equal under-standing of the role of architecture, and a number of studies have been performedthat quantified the relative effects of density and architecture. In one such study,104 specimens of human trabecular bone from a variety of anatomic sites weremachined and tested along anatomic axes (42), resulting in a large variation oftrabecular orientation across specimens. Correlations between elastic modulus inthe different anatomic directions and (a) bone volume fraction, (b) trabecular ori-entation (defined by the MIL values in the directions of the specimen geometricaxes), and (c) anisotropy ratio (defined from the ratio of maximum to minimumeigenvalues of the MIL ellipsoid) revealed that these variables combined explainedabout 90% of the observed variation in mechanical properties. In a similar seriesof studies on both bovine and human bone (25, 43), it was found that up to 94% ofthe observed variation in measured elastic modulus could be explained by a com-posite measure of bone volume fraction, trabecular orientation, and anisotropyratio (Figure 7a). Similarly, 94% of the variation in the 21 components of the
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 315
Figure 6 On-axis elastic modulus plotted as a function of apparent density for trabec-ular bone specimens taken from the human vertebral body (HVB) and bovine proximaltibia (BPT). (Adapted from Reference 55, with permission.)
elasticity tensor calculated using microstructural finite element models was cap-tured by volume fraction combined with fabric (41) (Figure 7b). In all these studies,bone volume fraction alone explained significantly less of the variance in elasticproperties. However, despite the highr2 values obtained in these studies, thereremains substantial scatter. For example, even with anr2 value of 0.94, values ofmodulus can vary by over 60% for a given value of the composite explanatoryvariable (25) (Figure 7a).
These results represent notable improvements in the ability of researchers topredict trabecular bone anisotropic elastic behavior, and the early consensus wasthat interspecimen variations in tissue properties were not important. However,as discussed above, the summary statistics suggest a level of precision that maynot be reflective of the true magnitude of the unexplained variance. The impor-tant issue now is to understand why some specimens can have such low valuesof modulus (and strength) at a given volume fraction. One plausible explanationis intra-specimen variations of tissue or structural properties within the specimen.A number of studies using nanoindentation to measure the tissue elastic modulus(68, 69) have indicated that substantial variations in tissue modulus can exist bothwithin and across specimens. The structure-function studies performed on com-puter models (17, 41) do not account for any effects of intraspecimen variations intissue properties and the biomechanical consequences of such variations remainunknown. Idealized computational models have shown that intraspecimen varia-tions in trabecular thickness can alter apparent modulus to the equivalent extentof 10 years of bone loss (70). It is expected that with the availability of power-ful analytical tools, we will soon understand the potentially important effects of
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
316 KEAVENY ET AL
Figure 7 (a) Regression of elastic modulus and a composite explanatory variable of densityand fabric provided a squared correlation coefficient of 0.94. However, for a given value ofthe composite explanatory variable, modulus varied by approximately 60%. (b) The completefabric tensor combined with volume fraction results in a similar level of correlation betweenpredicted and numerically calculated elastic properties. (Figure 7a adapted from Reference25, Figure 7b from Reference 41, with permission.)
intraspecimen variations in structure and material properties on the apparent levelbehavior. This in turn should provide substantial insight into why some specimenscan have low strength but normal levels of bone volume fraction and specimen-averaged measures of architecture.
STRENGTH
The same early studies that characterized the dependence of elastic modulus ondensity and site generally addressed strength as well and found similar trends.Namely, strength can vary by an order of magnitude across sites (Table 1), isanisotropic, and depends on density with either a linear or a power law relation.The density-architecture interaction is also an issue. Although Ciarelli et al (26)reported a dependence of the regression coefficients in the power law relation onanatomic site, no specific statistical comparisons were given, and no other studieshave compared strength across anatomic sites using the same test protocols andcontrolling for anisotropy for human bone. Thus, although substantial data fromseparate studies (Table 2) have provided a basis for biomechanical analyses ofwhole bones, additional work is required to provide more controlled comparisonsacross sites.
A further complexity with the strength behavior is the lower strength in tensioncompared with compression (67, 71), and the still lower strength in shear (72).
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 317
TABLE 2 Power law regressions between ultimate stress and apparent density forcompressive loading of human trabecular bone specimens from a range of anatomicsitesa
Cadavers σσ = AρρB
SpecimensStudy (reference) Number Age (No.) A B r2
Proximal tibia (63) 9 59–82 121 34.2 1.56 0.79
Proximal femur (133) 4 25–82 49 25.0 1.80 0.93
Lumbar spineHansson et al (134) 3 71–84 231 50.3 2.24 0.76Mosekilde et al (22) 42 15–87 40 24.9 1.80 0.83Kopperdahl & 11 32–65 22 33.2 1.53 0.68Keaveny (67)b
aσ , in megapascals;ρ, in grams per cubic centimeters.b0.2% offset yield stress is reported instead of ultimate stress because the latter was not measured.
These trends depend on the loading direction, and the relative differences in thesestrengths increase with increasing modulus (see Figure 4). Thus, from a strengthperspective, the properties of trabecular bone are heterogeneous (vary with age,site, disease, etc), anisotropic (depend on loading direction), and asymmetric (differin tension versus compression versus shear). Because most available data on suchcomplex characteristics of trabecular bone are for bovine tissue, there remains aneed to measure many of these characteristics for human tissue. Such data shouldimprove the ability of finite element models of whole bones to more accuratelypredict fracture loads for a variety of loading conditions, an important issue formany clinical problems.
Despite all this complexity, if failure is characterized by measures of strain, itcan be seen that nature may have provided a material that has remarkably simplefailure behavior. There is a strong linear correlation between the stress at whichtrabecular bone fails and the corresponding elastic modulus (e.g. 19, 20, 71, 73)(Figure 8). Because the ratio of stress to modulus is strain, at least for a linearlyelastic material, this correlation suggests that failure strains for trabecular bone arerelatively constant. Experiments that made direct measurements of the strains atfailure found that they have only a slight, if any, dependence on density (e.g. 67, 7174, 75). Also, the (0.2% offset) yield strains for bovine trabecular bone are higherin compression than tension (67, 71), and even more interesting, the yield strainsare isotropic (75, 76). Furthermore, yield strains are relatively uniform within eachsite but can vary across sites (67, 77). The simplicity of this strain-based descriptionof failure is extremely important in trabecular bone biomechanics. It implies, forexample, that if the elastic properties of trabecular bone are known, then regardlessof bone density, strength can be estimated with a high degree of accuracy for anyloading axes based only on the tensile and compressive yield strains.
Multiaxial behavior is important clinically because multiaxial stresses can occurduring falls, during trauma, and at the bone-implant interface. In contrast to the
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
318 KEAVENY ET AL
Figure 8 Yield stress versus modulus for bovine tibial trabecular bone, loaded inan on-axis configuration in tension and compression. (From Reference 71, with per-mission.)
multitude of studies on the uniaxial strength behavior of trabecular bone, few stud-ies have addressed multiaxial strength behavior (78–81). Two aspects of the mul-tiaxial behavior have been addressed experimentally: the response to axial-shearloads and the response to triaxial compressive loads. In addition, theoretical formu-lations have been proposed (80, 81) based on the Tsai-Wu quadratic theory, whichwas originally developed for engineering composite materials (82, 83), and oncellular solid theory (79). Collectively, these studies have shown that the Tsai-Wutheory, although a good choice for axial-shear loading, does not work well for triax-ial loading because indications are that the failure envelope for the latter does not fitthe ellipsoidal shape obtained from the quadratic formulation of the theory (80).
Based on the observation that many specimens failed during the triaxial testsat stresses close to their uniaxial values (80), a simple cellular solid analyticaltheory was developed for the special case of axial-shear loading (79). The re-sult was a triangular failure envelope, built on the assumption that at the levelof individual trabeculae, bending stresses would primarily develop in response tothe apparent level shear loads, and axial stresses would primarily develop in re-sponse to the apparent level axial loads. The theory was calibrated using data fromprevious uniaxial experiments, and the resulting predictions of the axial-shearbehavior matched the experimental data to within 7.7%. In addition, when theapplied stresses were normalized (divided by modulus), the effects of density dis-appeared and the behavior of all specimens could be described by a single criterion(Figure 9). This is an excellent performance considering the complexity of the tra-becular bone strength properties and the loading conditions. It is expected therefore
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 319
Figure 9 Axial-shear behavior of bovine proximal tibial trabecular bone. When thestress axes are normalized by modulus, data from many specimens fit a single failureenvelope. Both criteria shown were calibrated to fit through the uniaxial tension, com-pression, and shear failure points. (Gray bars)±1 standard deviation of those data.(From Reference 79, with permission.)
that the appropriate multiaxial failure criterion for trabecular bone should be basedon strains, and that it should allow for microstructural failure mechanisms that aredifferent and perhaps independent for the different loading directions.
POSTYIELD AND DAMAGE BEHAVIOR
A relatively new area of trabecular bone biomechanics is the damage behavior atboth apparent and tissue levels. Damage and repair of individual trabeculae arenow recognized as normal physiologic processes (84, 85) that tend to increasewith age (86–88), and which may have clinical and biological relevance. It hasbeen proposed that damage to trabeculae could increase osteoporotic fracture risk(73, 84, 89), act as a stimulus for remodeling (90), occur during implantation ofprostheses, particularly in the elderly spine where the bone is fragile (89), or beinvolved in aseptic necrosis of the femoral head, degenerative joint disease (91),and other bone disorders (92).
Experiments on machined specimens of bovine tibial (93, 94) and human ver-tebral (89) trabecular bone, as well as the whole vertebral body (95), have es-tablished that when trabecular bone is loaded past its yield point, it unloads to aresidual strain at zero stress, reloads with a modulus equal to its initial modulus, butthen develops a reduced modulus characteristic of a perfectly-damaging material(Figure 10). For human vertebral bone, residual strains of up to 1.05% occurwith compressive loading of up to 3.0% strain and increase in a slightly nonlinear
27 Jun 2001 12:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
320 KEAVENY ET AL
Figure 10 Load-unload-reload postyield behavior of human vertebral trabecularbone. For multiple specimens, the secant modulus (dashed line) is statistically sim-ilar to the slope of the main linear region in the reloading curve, and the initial slope ofthe reloading curve is statistically similar to the modulus of the initial loading cycle.(From Reference 89, with permission.)
fashion with increasing total applied strain (89). Clinical spine fractures are definedin terms of permanent deformations, but many fractures are not associated withany specific traumatic event (96, 97). Thus, it is possible that isolated overloads,or fatigue (see below), that do not cause overt fracture of the bone do cause subtlebut cumulative permanent deformations that may result in clinical fractures aftera number of years (95). This hypothesis remains to be tested.
The reductions in modulus and strength that occur for reloading after monotonicoverloading are substantial and depend strongly on the magnitude of the appliedstrain but are mostly independent of volume fraction. In an experiment performedon machined specimens of human vertebral trabecular bone (89), modulus reduc-tions (between the intact Young’s and the residual moduli) were over 85% forapplied apparent strains of up to 3.0% (Figure 11). Using concepts of continuumdamage mechanics for brittle materials (98), these modulus reductions can be in-terpreted as quantitative measures of effective mechanical damage in the specimen.Thus, a modulus reduction of 85% corresponds to 85% damage from a mechanicalperspective. Such behavior has also been found for bovine bone (93, 94) and forthe entire vertebral body (95) and appears therefore to be independent of density,anatomic site, and even species. Indeed, the similarity of this behavior with that ofcortical bone (99) suggests that the damage occurs at the nanometer scale of thecollagen and hydroxyapatite.
At the trabecular tissue level, examination of the physical damage that occurswith overloading (Figure 12) has confirmed that subtle damage within trabecu-lae (versus fracture of entire trabeculae) can cause large reductions in apparent
27 Jun 2001 12:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 321
Figure 11 Dependence of percent stiffness reduction on level of initial applied “plas-tic” strain for trabecular cores from the bovine proximal tibia and human vertebral body.(Adapted from Reference 95, with permission.)
modulus (100). Consistent with this, Fyhrie & Schaffler (73) reported that for hu-man vertebral specimens loaded in compression to 15% strain, the primary mech-anism of failure was microscopic cracking rather than overt fracture of individ-ual trabeculae. Complete fracture of trabeculae was confined to elements orientedtransversely to the loading direction. Laser scanning confocal microscopy (101) hasshown that cross-hatch shear band type staining and more diffuse (102) staining ob-served with basic fuchsin included “ultramicrocracks” about 10µm in length. Theimplication is that cracking can occur at very small scales. Zysset & Curnier (94)came to a similar conclusion after observing similarities in postyield and damagebehaviors between bovine trabecular bone they tested and cortical bone from theliterature.
CREEP AND FATIGUE
Following up on preliminary reports on time-dependent failure modes (103, 104),it has been demonstrated that bovine trabecular bone has fatigue and creep char-acteristics similar to those of cortical bone. The creep characteristics exhibit thethree classical phases of an initial rapid response, a steady state creep at a constantcreep rate, and a rapid increase in strain just before fracture (105). Cyclic loadingresults in cumulative creep deformations in addition to loss of stiffness (106, 107).Standard “S-N” (applied stress versus number of cycles to failure) (Figure 13) andcreep stress-time curves, using nondimensional measures of stress, have been re-ported that can serve as input into whole bone and bone-implant structural analyses.These curves indicate that the compressive strength of devitalized trabecular bonecan be reduced by up to 70% after 106 cycles of loading. Of course, in vitro creepor fatigue experiments preclude biological healing, and thus the resulting fatigue
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
322 KEAVENY ET AL
Figure 13 Traditional “S-N” fatigue curve for bovine trabecular bone for zero-compression cyclic loading. The applied stress1σ has been normalized by the initialmodulus Eo. (From Reference 107, with permission.)
S-N or creep stress-time curves might best be considered as lower bounds on thespecimen life, i.e. we can expect a longer life if biological healing of the fatigue-or creep-related damage occurs. However, it has been suggested that osteoclasticresorption during the remodeling process can in some situations reduce strength ifthe resulting Howslip’s lacunae serve as significant stress concentrations (108). Ifthat is the case, then the in vitro fatigue characteristics may represent upper boundson the in vivo fatigue life.
The creep and fatigue characteristics of human trabecular bone are not known.Tensile creep or fatigue characteristics, how any of these behaviors may depend onaging or disease, and how previous mechanical overloads or biological remodelingmay potentially accentuate fatigue damage are also not known. More important,all studies reported to date on trabecular bone have used relatively high loadinglevels that are probably outside the range of repetitive, habitual load levels. Thus,despite novel work over the past 10 years on characterization of the time-dependentbehavior of trabecular bone, there remains much to be done. This is particularlyrelevant to osteoporotic fractures in the spine that are typically not associated with aspecific trauma (96, 97). Accumulation of damage from overloads, fatigue, or creeploading may also help explain the 10%–20% of spontaneous hip fractures that occurwithout a fall, and why only about 2% of those who fall actually fracture their hips.
MICROMECHANICAL FINITE ELEMENT MODELING
Because of the complexity of the empirical data described above, a numberof computational studies have been performed to gain insight into the un-derlying micromechanics of the bone. In particular, two strategies have been
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 323
developed to perform micromechanical finite element analyses of trabecular bonespecimens.
The first class of models is based on the cellular solid paradigm. The strategy isto account for some of the complexity of trabecular architecture while maintain-ing the computational efficiency that allows for the development of an intuitiveunderstanding of the micromechanics. One method of idealizing the trabeculargeometry has been to incorporate the most salient features of the trabecular archi-tecture based on published histomorphometric studies. Models have incorporatedstatistical distributions of spacing, angular orientation, and thickness (70, 109). Al-ternatively, Voronoi techniques can be used to generate an idealized nonperiodicmesh representative of trabecular bone (110, 111).
Various lattice-type finite element models have been used to examine the ar-chitectural manifestations of aging. Using age-related regressions of mean thick-ness and spacing for vertebral trabecular bone, trends of elastic modulus reduc-tion with aging correlated reasonably well with experimental observations (109).Other analyses demonstrated the potentially important role of stark interruptionsin the trabecular network versus more uniform thinning (110, 112). Whereas thesestudies considered only randomly placed defects in the trabecular network, onestudy (113) reported on a more mechanistic basis for removal of trabeculae byconsidering both fatigue and creep. The study demonstrated that after reductionsof apparent modulus of about 15%, further removal of trabeculae from fatiguecrack propagation produced apparent modulus reductions that were greater thanfor random removal of trabeculae. This occurred presumably because of a stress-concentration effect of the accumlated fractures, or load redistribution around theaccumulated fractures, and overloading and accelerated failure of the adjacenttrabeculae.
The other class of microstructural finite element models, the high-resolutionmodel, represents a critical methodological advance in trabecular bone biomecha-nics. This technique uses a high-resolution, three-dimensional image of a specificspecimen at up to 10-µm spatial resolution. The strategy is to directly convert thedigital image into a finite element mesh, thereby avoiding the need to generatemore traditional isoparametric meshes of the topographically complex trabeculararchitecture. In the more popular implementation, a finite element mesh is gener-ated directly from a three-dimensional image by applying a one-to-one mapping ofimage voxels to eight-node hexahedral finite elements (114, 115) (Figure 14). Thismethod is particularly attractive for trabecular bone because an explicit mathemati-cal representation of the geometry is not required. The resulting models can containmillions of elements (116, 117). The congruence of the elements in the model isthen exploited to produce specialized solution algorithms that substantially reducememory requirements (118, 119). A number of studies have been reported thatcharacterize the numerical convergence behavior of this class of models, whereit is recommended that at least four elements span the mean trabecular thickness(120–122).
The mechanical properties of the trabecular tissue are usually assumed tobe homogeneous and isotropic within the high-resolution finite element model.
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
324 KEAVENY ET AL
Figure 14 A 20-µm, high-resolution image of a 5-mm cube specimen of bovine tibialtrabecular bone (left), and a portion of the resulting “high-resolution” finite element model(right). The finite element mesh (top front corner) is approximately 1.25 mm on each side,with an element size of approximately 60µm on a side.
Although recent studies using nanoindentation indicate that the tissue elastic mod-ulus may vary substantially throughout the specimen (68), it has been demonstratedthat the use of isotropic and homogeneous properties can result in good predictionsof the apparent elastic properties (123). Linear elasticity is also assumed, resultingin the overall stiffness of the model being a linear function of the assumed trabec-ular tissue modulus. Using an inverse technique, an “effective” modulus for thetrabecular tissue can be calculated by determining the ratio of the tissue to apparentelastic moduli predicted by the finite element results, and multiplying this ratioby the experimentally measured apparent modulus for the same specimen (115).Using this approach, a large range (mean values from 5.6–18.7 GPa) of effectivetissue moduli has been reported (123–126). Although this large range may reflectdifferences in anatomic site, age, and the experimental methods used to determinethe apparent elastic properties, a similar range of values has been reported usingother measurement techniques (for a review, see 127). Even so, recent nanoinden-tation studies for human bone have reported values of the tissue modulus in therange of only 11 (68) to 18 (128, 129) GPa, which suggests that the lower valuesreported for the effective tissue modulus may be more reflective of experimentalor computational artifacts. Thus, it remains a challenge to use the high-resolutionfinite element technique to estimate absolute values of tissue properties.
Because it is so difficult to measure the orthotropic elastic constants of tra-becular bone in a purely experimental setting, the high-resolution finite element
27 Jun 2001 12:4 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 325
method provides a powerful means to address this issue. Using the concept of arepresentative volume element (130), the 21 unique values in the apparent stiffnesstensor can be found from six uniaxial strain simulations. The calculation involvesno a priori assumption of the elastic symmetry of the bone and, in this sense, is su-perior to actual mechanical tests. Results are typically normalized by the assumedtissue modulus, thereby eliminating this variable from consideration. The resultingelastic stiffness matrix can be rotated into a coordinate system that minimizes thenonorthotropic terms in the elasticity tensor. Using this technique, three studiesperformed on bone taken from multiple sites have found that the orthotropic coef-ficients of the stiffness matrix were more than two orders of magnitude larger thanany remaining term (17, 32, 33), i.e. trabecular bone can be considered to haveorthotropic symmetry. As discussed earlier, this technique has also been used toprovide substantial insight into relationships between elastic modulus and bothvolume fraction and fabric, as well as determination of the principal material andstructural directions (17, 33, 131).
Most recently, the high-resolution finite element technique has been extendedto address failure properties (126). The main assumption used was that the tensileand compressive yield strains of the trabecular tissue were similar to those of cor-tical bone and were the same for all trabecular bone specimens. After calibratingthese properties for one specimen, and by using a specimen-specific value of effec-tive tissue modulus, the compressive, tensile, and shear yield apparent propertieswere predicted for six other specimens and were found to be statistically indis-tinguishable from those measured in previous experiments (71, 72). This levelof performance is outstanding considering that only two material constants wereused to characterize the failure properties for all specimens and loading directions.Thus, this technique has great potential for exploring strength behavior. For ex-ample, because it is difficult to perform real multiaxial experiments on sufficientnumbers of human trabecular bone specimens to account for heterogeneity, it islikely that the multiaxial strength problem will only be tractable if addressed usinga combined experimental-computational approach. Because the high-resolutionfinite element models are microstructurally based, they should be able to predictmultiaxial failure behavior without introducing any new assumptions. In addi-tion, these models can provide predictions of regions of tensile and compres-sive failure of tissue within the specimen (Figure 15). Such data now providea basis for addressing possible strain-specific biological responses to damage inbone, an important issue in understanding the mechano-biology of trabecular bone(84).
CONCLUDING REMARKS
The field of trabecular bone biomechanics has grown steadily over the past 30years and has reached a level of maturity resulting in substantial collaborations be-tween engineers, biologists, radiologists, and clinicians. We have reviewed some
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
326 KEAVENY ET AL
of the highlights of this work, mostly from a mechanics perspective, and haveattempted to note the important achievements and exciting areas of future work.Trabecular bone is highly heterogeneous in microstructure, which results in sub-stantial variations of elastic modulus and strength both within and across sites.Even so, the strains at which the bone fails are relatively constant, suggesting abiological control mechanism targeted toward apparent strains. Trabecular bone isalso anisotropic, and sophisticated computer modeling and micron-level imagingtechniques have been developed that provide much insight into the relationshipsbetween such aniostropy and the underlying architecture and how these relationsmay change with aging, disease, and drug treatment. The same types of modelspromise the ability to better understand the role of damage in degradation of me-chanical properties and offer a way to tackle so far intractable problems such asthe multiaxial strength behavior.
It should be noted that we did not review many areas that are important totrabecular bone biomechanics2, namely, the role of trabecular bone mechanicalbehavior in whole bone mechanics nor did we review any biological effects ex-plicitly. Examples of the latter include bone adaptation, tissue engineering, and thein vivo response to drug treatments, disuse, space flight, and exercise regimens.These are important areas intimately related to the subject matter reviewed here.
In terms of understanding the clincal effects of aging and disease, a numberof challenges remain within the domain of trabecular bone mechanics. Most im-portantly perhaps, we need to understand how some trabecular bone can be soweak, or so susceptible to fatigue failure, despite having normal levels of bonedensity, and perhaps even a normal measure of architecture (by current standardsof the latter). Indeed, this may constitute a more rigorous definition of osteoporoticbone as current definitions cannot predict who will fracture with much precision.Factors such as damage accumulation, possible changes in tissue ducitility fromchanges in the collagen, alterations in the biological remodeling dynamics, andvarious types of intraspecimen variations in material and structural properties arecurrently under investigation. These studies will require new types of model inputsuch as statistical distributions of tissue mineralization, damage, and trabecularthickness, as well as new assays of collagen and mineral status and perhaps theirspatial distribution. Knock-out animal models also promise a way to alter the sys-tem to produce a specific phenotype characteristic of aging or disease and thisstrategy is becoming more widespread.
At the whole bone level, a number of issues remain. The relative role of thecortical shell versus trabecular bone remains controversial for bones with thincortices such as the vertebral body. The effects of trabecular anisotropy on wholebone behavior in bones such as the proximal femur remain unknown. Multiaxial
2Although a biologist might argue that we have reviewed the mechanics as opposed to thebiomechanics of trabecular bone in this review because we have not addressed biology perse, the engineering perspective accepts the term biomechanics as we are addressing themechanics of a biological system.
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 327
failure criteria need to be developed to enable fracture analysis of whole bonesfor such loading conditions as a fall, sports impact, or vehicular trauma and formore detailed analysis of bone-implant systems. Understanding the developmentof damage over time in whole bones from creep, repetitive loading, or intermittentfalls—and the biological responses to such damage—represents a complex struc-tural analysis, requiring development of time-dependent damage accumulation andbiological repair constitutive models for the trabecular bone and correspondinglylarge scale, materially nonlinear and time-dependent finite element models. Thein vivo animal experiments required to validate such models also present technicalchallenges.
In the future, we expect that with the substantial body of current knowledgeabout trabecular bone mechanics, and with the highly sophisticated experimental-computational techniques that have been developed, there will be significant ad-vances made in many areas both at the tissue and whole bone levels. The fieldof trabecular bone biomechanics is intensely rich. We believe that the best is yetto come and that the key to continued successlies in multidisciplinary approachesthat can successfully address the complexity of this important biological tissue
ACKNOWLEDGMENTS
The authors acknowledge support from the National Institutes of Health (grantsAR41481, AR43784), the National Science Foundation (grant BES-9625030 anda Graduate Student Fellowship), and the Miller Institute for Basic Research inScience, Berkeley. Computer resources were provided in part by the NationalPartnership for Advanced Computing Infrastructure and Lawrence LivermoreNational Laboratory (ISCR B291837, 97-06 and 98-04).
Visit the Annual Reviews home page at www.AnnualReviews.org
LITERATURE CITED
1. Goldstein SA. 1987. The mechanicalproperties of trabecular bone: dependenceon anatomic location and function.J.Biomech.20:1055–61
2. Keaveny TM, Hayes WC. 1993. A 20-year perspective on the mechanical pro-perties of trabecular bone.J. Biomech.Eng.15:534–42
3. Linde F. 1994. Elastic and viscoelas-tic properties of trabecular bone by acompression testing approach.Dan. Med.Bull. 41:119–38
4. Keyak JH, Rossi SA. 2000. Prediction offemoral fracture load using finite element
models: an examination of stress- andstrain-based failure theories.J. Biomech.33:209–14
5. Keyak JH, Rossi SA, Jones KA, SkinnerHB. 1998. Prediction of femoral fractureload using automated finite element mo-deling.J. Biomech.31:125–33
6. Keyak JH, Fourkas MG, Meagher JM,Skinner HB. 1993. Validation of an auto-mated method of 3-dimensional finite ele-ment modeling of bone.J. Biomed. Eng.15:505–9
7. Skinner HB, Kilgus DJ, Keyak J,Shimaoka EE, Kim AS, et al. 1994.
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
328 KEAVENY ET AL
Correlation of computed finite elementstresses to bone density after remodel-ing around cementless femoral implants.Clin. Orthop.305:178–89
8. Cody DD, Gross GJ, Hou FJ, SpencerHJ, Goldstein SA, et al. 1999. Femoralstrength is better predicted by finite el-ement models than QCT and DXA.J.Biomech.32:1013–20
9. Faulkner KG, Cann CE, Hasegawa BH.1991. Effect of bone distribution on ver-tebral strength: assessment with a patient-specific nonlinear finite element analysis.Radiology179:669–74
10. Silva MJ, Keaveny TM, Hayes WC.1998. Computed tomography-based finiteelement analysis predicts failure loads andfracture patterns for vertebral sections.J.Orthop. Res.16:300–8
11. Keaveny TM, Bartel DL. 1995. Mechan-ical consequences of bone ingrowth in ahip prosthesis inserted without cement.J.Bone Jt. Surg. Am.77:911–23
12. Lotz JC, Cheal EJ, Hayes WC. 1995.Stress distributions within the proximalfemur during gait and falls: implicationsfor osteoporotic fracture.Osteoporos. Int.5:252–61
13. Lotz JC, Cheal EJ, Hayes WC. 1991.Fracture prediction for the proximal femurusing finite element models. Part I: Lin-ear analysis.J. Biomech. Eng.113:353–60
14. Lotz JC, Cheal EJ, Hayes WC. 1991.Fracture prediction for the proximal femurusing finite element models. Part II: Non-linear analysis.J. Biomech. Eng.113:361–65
15. Gibson LJ, Ashby MF. 1997.CellularSolids: Structures and Properties. Ox-ford, UK: Pergamon. 510 pp. 2nd ed.
16. Harrigan TP, Jasty M, Mann RW, Har-ris WH. 1988. Limitations of the conti-nuum assumption in cancellous bone.J.Biomech.21:269–75
17. Zysset PK, Goulet RW, Hollister SJ.1998. A global relationship between tra-becular bone morphology and homoge-
nized elastic properties.J. Biomech. Eng.120:640–46
18. Choi K, Goldstein SA. 1992. A compa-rison of the fatigue behavior of humantrabecular and cortical bone tissue.J.Biomech.25:1371–81
19. Goldstein SA, Wilson DL, SonstegardDA, Matthews LS. 1983. The mechani-cal properties of human tibial trabecularbone as a function of metaphyseal loca-tion. J. Biomech.16:965–69
20. Brown TD, Ferguson AB. 1980. Mechan-ical property distributions in the cancel-lous bone of the human proximal femur.Acta Orthop. Scand.51:429–37
21. McCalden RW, McGeough JA, Court-Brown CM. 1997. Age-related changesin the compressive strength of cancellousbone. The relative importance of changesin density and trabecular architecture.J.Bone Joint Surg. Am.79:421–27
22. Mosekilde L, Mosekilde L, DanielsenCC. 1987. Biomechanical competence ofvertebral trabecular bone in relation toash density and age in normal individu-als.Bone8:79–85
23. Mosekilde L, Mosekilde L. 1986. Nor-mal vertebral body size and compressivestrength: relations to age and to verte-bral and iliac trabecular bone compressivestrength.Bone7:207–12
24. Ding M, Dalstra M, Danielsen CC, Ka-bel J, Hvid I, et al. 1997. Age variations inthe properties of human tibial trabecularbone.J. Bone Joint Surg. Br.79:995–1002
25. Hodgskinson R, Currey JD. 1990. Theeffect of variation in structure on theYoung’s modulus of cancellous bone: acomparison of human and nonhuman ma-terial.Proc. Inst. Mech. Eng.204:115–21
26. Ciarelli MJ, Goldstein SA, Kuhn JL,Cody DD, Brown MB. 1991. Evaluationof orthogonal mechanical properties anddensity of human trabecular bone from themajor metaphyseal regions with materialstesting and computed tomography.J. Or-thop. Res.9:674–82
27. Williams JL, Lewis JL. 1982. Properties
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 329
and an anisotropic model of cancellousbone from the proximal tibial epiphysis.J. Biomed. Eng.104:50–56
28. Galante J, Rostoker W, Ray RD. 1970.Physical properties of trabecular bone.Calcif. Tissue Res.5:236–46
29. Wolff J. 1892.Das Gesetz der Transfor-mation der Knochen. Berlin: Hirschwald
30. Wolff J. 1986.The Law of Bone Remod-elling Berlin/New York: Springer-Verlag.126 pp.
31. Cowin SC. 1986. Wolff’s Law of trabec-ular architecture at remodeling equilib-rium. J. Biomech. Eng.108:83–88
32. Yang G, Kabel J, Van Rietbergen B,Odgaard A, Huiskes R, et al. 1999. Theanisotropic hooke’s law for cancellousbone and wood.J. Elasticity53:125–46
33. Odgaard A, Kabel J, an Rietbergen B,Dalstra M, Huiskes R. 1997. Fabric andelastic principal directions of cancellousbone are closely related.J. Biomech.30:487–95
34. Turner CH, Cowin SC, Rho JY, AshmanRB, Rice JC. 1990. The fabric depen-dence of the orthotropic elastic constantsof cancellous bone.J. Biomech.23:549–61
35. Turner CH, Cowin SC. 1988. Errors in-troduced by off-axis measurements of theelastic properties of bone.J. Biomech.110:213–14
36. Whitehouse WJ. 1974. The quantita-tive morphology of anisotropic trabecularbone.J. Microsc.2:153–68
37. Whitehouse WJ. 1974. A stereologi-cal method for calculating internal sur-face areas in structures which have be-come anisotropic as the result of linearexpansions or contractions.J. Microsc.101(2):169–76
38. Harrigan T, Mann R. 1984. Characteriza-tion of microstructural anisotropy in or-thotropic materials using a second ranktensor.J. Mater. Sci.19:761–67
39. Cowin SC. 1985. The relationship be-tween the elasticity tensor and the fabrictensor.Mech. Mater.4:137–47
40. Cowin SC, Turner CH. 1992. On the rela-tionship between the orthotropic Young’smoduli and fabric.J. Biomech.25:1493–94
41. Kabel J, van Rietbergen B, Odgaard A,Huiskes R. 1999. Constitutive relation-ships of fabric, density, and elastic proper-ties in cancellous bone architecture.Bone25:481–86
42. Goulet RW, Goldstein SA, Ciarelli MJ,Kuhn JL, Brown MB, et al. 1994. The re-lationship between the structural and or-thogonal compressive properties of tra-becular bone.J. Biomech.27:375–89
43. Hodgskinson R, Currey JD. 1990. Effectsof structural variation on Young’s modu-lus of nonhuman cancellous bone.Proc.Inst. Mech. Eng.204:43–52
44. Turner CH. 1992. On Wolff’s law of tra-becular architecture.J. Biomech.25:1–9
45. Kuhn JL, Goldstein SA, Feldkamp LA,Goulet RW, Jesion G. 1990. Evaluationof a microcomputed tomography systemto study trabecular bone structure.J. Or-thop. Res.8:833–42
46. Kinney JH, Lane NE, Haupt DL. 1995.In vivo, three-dimensional microscopyof trabecular bone.J. Bone Miner. Res.10:264–70
47. Ruegsegger P, Koller B, M¨uller R. 1996.A microtomographic system for the non-destructive evaluation of bone architec-ture.Calcif. Tissue Int.58:24–29
48. Majumdar S, Kothari M, Augat P, New-itt DC, Link TM, et al. 1998. High-res-olution magnetic resonance imaging:three-dimensional trabecular bone archi-tecture and biomechanical properties.Bone22:445–54
49. Hipp JA, Jansujwicz A, Simmons CA,Snyder BD. 1996. Trabecular bone mor-phology from micro-magnetic resonanceimaging.J. Bone Miner. Res.11:286–97
50. Odgaard A, Andersen K, Melsen F, Gun-dersen HJ. 1990. A direct method for fastthree-dimensional serial reconstruction.J.Microsc.159:335–42
51. Beck JD, Canfield BL, Haddock SM,
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
330 KEAVENY ET AL
Chen TJH, Kothari M, et al. 1997. Three-dimensional imaging of trabecular boneusing the Computer Numerically Con-trolled Milling technique.Bone21:281–87
52. Odgaard A, Jensen EB, Gundersen HJ.1990. Estimation of structural anisotropybased on volume orientation. A new con-cept.J. Microsc.157:149–62
53. Odgaard A. 1997. Three-dimensionalmethods for quantification of cancellousbone architecture.Bone20:315–28
54. Cruz-Orive L, Karlsson L, Larsen S,Wainschtein F. 1992. Characterizing str-uctural anisotropy: a new concept.MicronMicrosc. Acta23:75–76
55. Keaveny TM. 1997. Mechanistic appro-aches to analysis of trabecular bone.Forma12:267–75
56. Zysset PK, Curnier A. 1995. An alterna-tive model for anisotropic elasticity basedon fabric tensors.Mech. Mater.21:243–50
57. Keaveny TM, Borchers RE, Gibson LJ,Hayes WC. 1993. Trabecular bone modu-lus and strength can depend on specimengeometry.J. Biomech.26:991–1000
58. Keaveny TM, Borchers RE, Gibson LJ,Hayes WC. 1993. Theoretical analysisof the experimental artifact in trabecularbone compressive modulus.J. Biomech.26:599–607
59. Keaveny TM, Pinilla TP, Crawford RP,Kopperdahl DL, Lou A. 1997. Systematicand random errors in compression test-ing of trabecular bone.J. Orthop. Res.15:101–10
60. Linde F, Hvid I, Madsen F. 1992. The ef-fect of specimen geometry on the mechan-ical behaviour of trabecular bone speci-mens.J. Biomech.25:359–68
61. Odgaard A, Linde F. 1991. The underes-timation of Young’s modulus in compres-sive testing of cancellous bone specimens.J. Biomech.24:691–98
62. Odgaard A, Hvid I, Linde F. 1989. Com-pressive axial strain distributions in can-cellous bone specimens.J. Biomech.22:829–35
63. Linde F, Hvid I. 1989. The effect of con-straint on the mechanical behaviour oftrabecular bone specimens.J. Biomech.22:485–90
64. Jacobs CR, Davis BR, Rieger CJ, Fran-cis JJ, Saad M, et al. 1999. The impact ofboundary conditions and mesh size on theaccuracy of cancellous bone tissue modu-lus determination using large scale finiteelement modeling.J. Biomech.32:1159–64
65. Carter DR, Hayes WC. 1977. The com-pressive behavior of bone as a two-phaseporous structure.J. Bone Joint Surg. Am.59:954–62
66. Rice JC, Cowin SC, Bowman JA. 1988.On the dependence of the elasticity andstrength of cancellous bone on apparentdensity.J. Biomech.21:155–68
67. Kopperdahl DL, Keaveny TM. 1998.Yield strain behavior of trabecular bone.J. Biomech.31:601–8
68. Zysset PK, Guo XE, Hoffler CE, MooreKE, Goldstein SA. 1999. Elastic modu-lus and hardness of cortical and trabecu-lar bone lamellae measured by nanoinden-tation in the human femur.J. Biomech.32:1005–12
69. Rho JY, Tsui TY, Pharr GM. 1997. Elasticproperties of human cortical and trabecu-lar lamellar bone measured by nanoinden-tation.Biomaterials18:1325–30
70. Yeh OC, Keaveny TM. 1999. Biome-chanical effects of intra-specimen varia-tions in trabecular architecture: a three-dimensional finite element study.Bone25:223–28
71. Keaveny TM, Wachtel EF, Ford CM,Hayes WC. 1994. Differences betweenthe tensile and compressive strengths ofbovine tibial trabecular bone depend onmodulus.J. Biomech.27:1137–46
72. Ford CM, Keaveny TM. 1996. The de-pendence of shear failure properties ofbovine tibial trabecular bone on appar-ent density and trabecular orientation.J.Biomech.29:1309–17
73. Fyhrie DP, Schaffler MB. 1994. Failure
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 331
mechanisms in human vertebral cancel-lous bone.Bone15:105–9
74. Hvid I, Bentzen SM, Linde F, MosekildeL, Pongsoipetch B. 1989. X-ray quantita-tive computed tomography: the relationsto physical properties of proximal tibialtrabecular bone specimens.J. Biomech.22:837–44
75. Turner CH. 1989. Yield behavior of bovi-ne cancellous bone.J. Biomech. Eng.111:256–60
76. Chang WCW, Christensen TM, PinillaTP, Keaveny TM. 1999. Isotropy of uni-axial yield strains for bovine trabecularbone.J. Orthop. Res.17:582–85
77. Morgan EF, Arramon YP, KopperdahlDL, Keaveny TM. 1999. Dependenceof yield strain on anatomic site for hu-man trabecular bone.Adv. Bioeng.43:23–24
78. Stone JL, Beaupre GS, Hayes WC. 1983.Multiaxial strength characteristics of tra-becular bone.J. Biomech.16:743–52
79. Fenech CM, Keaveny TM. 1999. A cellu-lar solid criterion for predicting the axial-shear failure properties of trabecular bone.J. Biomech. Eng.121:414–22
80. Keaveny TM, Wachtel EF, Zadesky SP,Arramon YP. 1999. Application of theTsai-Wu quadratic multiaxial failure cri-terion to bovine trabecular bone.J.Biomech. Eng.121:99–107
81. Cowin SC. 1989.Bone Mechanics. BocaRaton, FL: CRC. 313 pp.
82. Tsai S, Wu E. 1971. A general theory forstrength of anisotropic materials.J. Com-put. Mater.5:58–80
83. Wu E. 1974. Phenomenological anisotro-pic failure criterion. InMechanics of Com-posite Materials, ed. G Sendecky, pp.353–431. New York: Academic
84. Burr DB, Forwood MR, Fyhrie DP, Mar-tin RB, Schaffler MB, et al. 1997. Bonemicrodamage and skeletal fragility in os-teoporotic and stress fractures.J. BoneMiner. Res.12:6–15
85. Hansson T, Roos B. 1981. Microcallusesof the trabeculae in lumbar vertebrae and
their relation to the bone mineral content.Spine6:375–80
86. Hahn M, Vogel M, Amling M, Ritzel H,Delling G. 1995. Microcallus formationsof the cancellous bone: a quantitative anal-ysis of the human spine.J. Bone Miner.Res.10:1410–16
87. Mori S, Harruff R, Ambrosius W, BurrDB. 1997. Trabecular bone volume andmicrodamage accumulation in the femo-ral heads of women with and withoutfemoral neck fractures.Bone21:521–26
88. Fazzalari NL, Forwood MR, Smith K,Manthey BA, Herreen P. 1998. Assess-ment of cancellous bone quality in severeosteoarthrosis: bone mineral density, me-chanics, and microdamage.Bone22:381–88
89. Keaveny TM, Wachtel EF, KopperdahlDL. 1999. Mechanical behavior of humantrabecular bone after overloading.J. Or-thop. Res.17:346–53
90. Pugh JW, Rose RM, Radin EL. 1973. Apossible mechanism of Wolff’s law: tra-becular microfractures.Arch. Int. Physiol.Biochim.81:27–40
91. Benaissa R, Uhthoff HK, Mercier P.1989. Repair of trabecular fatigue frac-tures. Cadaver studies of the upper femur.Acta Orthop. Scand.60:585–89
92. Ohtani T, Azuma H. 1984. Trabecular mi-crofractures in the acetabulum. Histologicstudies in cadavers.Acta Orthop. Scand.55:419–22
93. Keaveny TM, Wachtel EF, Guo XE,Hayes WC. 1994. Mechanical behaviorof damaged trabecular bone.J. Biomech.27:1309–18
94. Zysset PK, Curnier A. 1996. A 3Ddamage model for trabecular bone basedon fabric tensors.J. Biomech.29:1549–58
95. Kopperdahl DL, Pearlman JL, KeavenyTM. 2000. Biomechanical consequencesof an isolated overload on the human ver-tebral body.J. Orthop. Res.18:685–90
96. Myers ER, Wilson SE, Greenspan SL.1996. Vertebral fractures in the elderly
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
332 KEAVENY ET AL
occur with falling and bending.J. BoneMiner. Res.11:S355
97. Cooper C, Atkinson EJ, O’Fallon WM,Melton LJ. 1992. Incidence of clini-cally diagnosed vertebral fractures: apopulation-based study in Rochester,Minnesota, 1985–1989.J. Bone Miner.Res.7:221–27
98. Krajcinovic D, Lemaitre J. 1987.Con-tinuum Damage Mechanics: Theory andApplications. New York: Springer-Verlag.294 pp.
99. Fondrk M, Bahniuk E, Davy DT, Micha-els C. 1988. Some viscoplastic character-istics of bovine and human cortical bone.J. Biomech.21:623–30
100. Wachtel EF, Keaveny TM. 1997. Depen-dence of trabecular damage on mechani-cal strain.J. Orthop. Res.15:781–87
101. Fazzalari NL, Forwood MR, MantheyBA, Smith K, Kolesik P. 1998. Three-dimensional confocal images of micro-damage in cancellous bone.Bone23:373–78
102. Vashishth D, Koontz J, Qiu SJ, Lundin-Cannon D, Yeni YN, et al. 2000. In vivodiffuse damage in human vertebral trabec-ular bone.Bone26:147–52
103. Schoenfeld CM, Lautenschlager EP, Me-yer PRJ. 1974. Mechanical properties ofhuman cancellous bone in the femoralhead.Med. Biol. Eng.12:313–17
104. Zilch H, Rohlmann A, Bergmann G,Kolbel R. 1980. Material properties offemoral cancellous bone in axial loading.Part II: Time dependent properties.Arch.Orthop. Trauma Surg.97:257–62
105. Bowman SM, Keaveny TM, Gibson LJ,Hayes WC, McMahon TA. 1994. Com-pressive creep behavior of bovine trabec-ular bone.J. Biomech.27:301–10
106. Michel MC, Guo XD, Gibson LJ, McMa-hon TA, Hayes WC. 1993. Compressivefatigue behavior of bovine trabecularbone.J. Biomech.26:453–63
107. Bowman SM, Guo XE, Cheng DW,Keaveny TM, Gibson LJ, et al. 1998.Creep contributes to the fatigue behav-
ior of bovine trabecular bone.J. Biomech.Eng.120:647–54
108. Martin RB, Gibson VA, Stover SM,Gibeling JC, Griffin LV. 1997. Residualstrength of equine bone is not reduced byintense fatigue loading: implications forstress fracture.J. Biomech.30:109–14
109. Jensen KS, Mosekilde L, MosekildeL. 1990. A model of vertebral trabecu-lar bone architecture and its mechanicalproperties.Bone11:417–23
110. Silva MJ, Gibson LJ. 1997. Modeling themechanical behavior of vertebral trabec-ular bone: effects of age-related changesin microstructure.Bone21:191–99
111. Silva MJ, Gibson LJ. 1997. The effectsof nonperiodic microstructure and de-fects on the compressive strength of two-dimensional cellular solids.Int. J. Mech.Sci.39:549–63
112. Vajjhala S, Kraynik AM, Gibson LJ.2000. A cellular solid model for modu-lus reduction due to resorption of trabec-ulae in bone.J. Biomech. Eng.122:511–15
113. Guo XE, McMahon TA, Keaveny TM,Hayes WC, Gibson LJ. 1994. Finite el-ement modeling of damage accumulationin trabecular bone under cyclic loading.J.Biomech.27:145–55
114. Hollister SJ, Brennan JM, Kikuchi N.1994. A homogenization sampling pro-cedure for calculating trabecular bone ef-fective stiffness and tissue level stress.J.Biomech.27:433–44
115. Van Rietbergen B, Weinans H, HuiskesR, Odgaard A. 1995. A new method todetermine trabecular bone elastic proper-ties and loading using micromechanicalfinite element models.J. Biomech.28:69–81
116. Ulrich D, van Rietbergen B, Laib A,Ruegsegger P. 1999. Load transfer anal-ysis of the distal radius from in vivohigh-resolution CT-imaging.J. Biomech.32:821–28
117. Van Rietbergen B, M¨uller R, UlrichD, Ruegsegger P, Huiskes R. 1999.
19 Jun 2001 13:3 AR AR136-12.tex AR136-12.SGM ARv2(2001/05/10)P1: GJB
BIOMECHANICS OF TRABECULAR BONE 333
Tissue stresses and strain in trabecu-lae of a canine proximal femur can bequantified from computer reconstruc-tions.J. Biomech.32:165–73
118. Van Rietbergen B, Weinans H, HuiskesR, Polman BJW. 1996. Computationalstrategies for iterative solutions of largeFEM applications employing voxel data.Int. J. Number Method Eng.39:2743–67
119. Hughes T, Ferencz R, Hallquist J. 1987.Large-scale vectorized implicit calcula-tion in solid mechanics on a Cray X-MP/48 utilizing EBE preconditioned con-jugate gradients.Comput. Methods Appl.Mech. Eng.61:215–48
120. Niebur GL, Yuen JC, Hsia AC, KeavenyTM. 1999. Convergence behavior of high-resolution finite element models of trabec-ular bone.J. Biomech. Eng.121:629–35
121. Ladd AJ, Kinney JH. 1998. Numericalerrors and uncertainties in finite-elementmodeling of trabecular bone.J. Biomech.31:941–45
122. Guldberg RE, Hollister SJ, CharrasGT. 1998. The accuracy of digital image-based finite element models.J. Biomech.Eng.120:289–95
123. Kabel J, van Rietbergen B, Dalstra M,Odgaard A, Huiskes R. 1999. The roleof an effective isotropic tissue modulus inthe elastic properties of cancellous bone.J. Biomech.32:673–80
124. Hou FJ, Lang SM, Hoshaw SJ, ReimannDA, Fyhrie DP. 1998. Human vertebralbody apparent and hard tissue stiffness.J.Biomech.31:1009–15
125. Ladd AJ, Kinney JH, Haupt DL, Gold-stein SA. 1998. Finite-element modelingof trabecular bone: comparison with me-chanical testing and determination of tis-sue modulus.J. Orthop. Res.16:622–28
126. Niebur GL, Feldstein MJ, Yuen JC, ChenTJ, Keaveny TM. 2000. High-resolution
finite element models with tissue strengthasymmetry accurately predict failure oftrabecular bone.J. Biomech.33:1575–83
127. Rho JY, Ashman RB, Turner CH. 1993.Young’s modulus of trabecular and cor-tical bone material: ultrasonic and mi-crotensile measurements.J. Biomech.26:111–19
128. Rho JY, Kuhn-Spearing L, Zioupos P.1998. Mechanical properties and the hi-erarchical structure of bone.Med. Eng.Phys.20:92–102
129. Turner CH, Rho J, Takano Y, TsuiTY, Pharr GM. 1999. The elastic prop-erties of trabecular and cortical bone tis-sues are similar: results from two mi-croscopic measurement techniques.J.Biomech.32:437–41
130. Van Rietbergen B, Odgaard A, KabelJ, Huiskes R. 1996. Direct mechanics as-sessment of elastic symmetries and prop-erties of trabecular bone architecture.J.Biomech.29:1653–57
131. Van Rietbergen B, Odgaard A, KabelJ, Huiskes R. 1998. Relationships be-tween bone morphology and bone elasticproperties can be accurately quantified us-ing high-resolution computer reconstruc-tions.J. Orthop. Res.16:23–28
132. Rohlmann A, Zilch H, Bergmann G,Kolbel R. 1980. Material properties offemoral cancellous bone in axial loading.Part I: time independent properties.Arch.Orthop. Trauma Surg.97:95–102
133. Lotz JC, Gerhart TN, Hayes WC. 1990.Mechanical properties of trabecular bonefrom the proximal femur: a quantitativeCT study. J. Comput. Assist. Tomogr.14:107–14
134. Hansson TH, Keller TS, Panjabi MM.1987. A study of the compressive proper-ties of lumbar vertebral trabeculae: effectsof tissue characteristics.Spine12:56–62
P1: FQP
June 26, 2001 16:15 Annual Reviews AR136-12-COLOR
Figure 12 Various types of physical damage patterns observed in bovine trabecular boneviewed with light microscopy at 200X magnification: (a) transverse cracks; (b) shear bands;(c) parallel cracks; and (d) complete fracture. (e) Diffuse damage in human vertebral trabe-cular bone viewed under polarized light and using basic fuchsin stain. (Pattern 1) Uniformstaining of a trabecular packet on the bone surface; (pattern 2) diffuse staining characterizedby linear and cross-hatched streaks. (f ) Pattern 2 shown at higher magnification underconfocal microscopy. (Figure 12a–d from Reference 100; Figure 12e,f from Reference102, with permission.)
P1: FQP
June 26, 2001 16:15 Annual Reviews AR136-12-COLOR
Fig
ure
15T
hece
ntra
l3-m
mcu
bic
port
ion
ofa
high
-res
olut
ion
fini
teel
emen
tmod
elof
abo
vine
tibia
ltra
becu
lar
bone
spec
imen
shad
edto
show
regi
ons
ofyi
elde
dtis
sue
atth
eap
pare
ntte
nsile
(lef
t)an
dco
mpr
essi
ve(r
ight
)0.
2%of
fset
yiel
dpo
ints
.(R
ed)
Reg
ions
exce
edin
gth
ete
nsile
tissu
eyi
eld
stra
in;(
blue
)re
gion
sex
ceed
ing
the
com
pres
sive
tissu
eyi
eld
stra
in.(
From
Ref
eren
ce12
6,w
ithpe
rmis
sion
.)