Biogeochemical Cycles: Natural Cycles of Elements
-
Upload
michael-payne -
Category
Education
-
view
309 -
download
3
description
Transcript of Biogeochemical Cycles: Natural Cycles of Elements
- 1. Biogeochemical Cycles: Natural Cycles of Elements Water, Nitrogen and Carbon
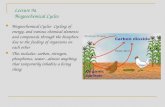
2. Cycles of Elements Nutrients (water, nitrogen, carbon) are cycled through the Earth in cycles called the BIOGEOCHEMICAL CYCLES. Law of Conservation of Mass Matter cannot be created or destroyed in chemical reactions it simply changes forms No new sources of matter on Earth instead it continuously cycles through Matter simply changes in the way that the elements are bonded together 3. I. Water Cycle 4. 5 Main Processes of the Water Cycle 1) Evaporation: Water turning from a liquid into a gas. Liquid water from oceans, lakes or other bodies of water, changes into water vapor (gas) and is released in the Water Cycle 5. 2) Transpiration: when liquid water in plants changes into water vapor (gas) and is released into the atmosphere Part of photosynthesis 3) Condensation: water vapor (gas) changes into liquid Ex. Cloud formation, dew 6. 4) Precipitation: when water returns to the Earths surface Water droplets get too large, they fall out of the air. Ex. Rain, snow, hail 5)Runoff- water moving from a higher elevation (mountains, etc.) to a lower elevation (oceans, lakes, seas, etc.) 7. The Water Cycle Transpiration Evaporation Precipitation Condensation Runoff 8. II. Nitrogen Cycle Nitrogen gas (N2) is naturally found in the atmosphere It is the most abundant gas in our atmosphere. N2 is unusable to most living things 9. Some bacteria and lightning are able to change nitrogen gas (N2) into chemicals called nitrates (NO3), which are usable by plants. 10. Nitrogen (NO3) in plants is passed on to an organism when it is eaten 11. Nitrogen (NO3) is returned to the soil by excretion from animals 12. Or when the animal dies 13. The nitrogen (NO3) in the soil is changed back into nitrogen gas (N2) by bacteria that live in the soil 14. III. Carbon Cycle 15. Carbon is found in the atmosphere as Carbon Dioxide (CO2) CO2 is used by plants during photosynthesis to make food and give off O2. 16. Animals eat food (plants) for energy. During cellular respiration (that takes place in plants and animals), the organism takes in O2 gives off CO2. This releases the CO2 back into the atmosphere (respiration). 17. Some carbon is trapped in dead creatures and changed into a fossil fuel (coal or oil). The decomposition of dead organisms releases carbon back to the atmosphere. 18. Burning of plant material or fossil fuels, also returns carbon as CO2 back to the atmosphere. 19. The Carbon Cycle 20. Greenhouse Effect Naturally occurring gasses in the Earths atmosphere that trap heat. They keep the Earths temperature about 60 warmer than it would be otherwise. Human Impact: Change: Increased level of greenhouse gases (such as CO2, methane, & water vapor) Cause: Burning of fossil fuels and deforestation Effect: Global warming 21. Ozone Layer The ozone layer is a naturally occurring layer of the stratosphere that absorbs large amounts of ultraviolet rays. Human Impact: Change: Reduction of ozone (O3) detected near poles Cause: Release of chlorofluorocarbons (CFC) that catalyze reactions with ozone molecules Effect: Increased ultraviolet radiation at the Earths surface