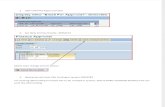
AVADO PFS Analysis (ITT Population) All P values vs. placebo Adapted from Miles et al. ASCO 2008,...
-
Upload
laura-wilkerson -
Category
Documents
-
view
212 -
download
0
Transcript of AVADO PFS Analysis (ITT Population) All P values vs. placebo Adapted from Miles et al. ASCO 2008,...

AVADO PFS Analysis (ITT Population)
All P values vs. placebo
Adapted from Miles et al. ASCO 2008, abstract LBA 1011.

AVADO: Response (patients with measurable disease), %
†mg/kg q3w
Adapted from Miles et al. ASCO 2008, abstract LBA 1011.

E2100 and AVADO Serious Adverse Events (%)
*ATE: arterial thromboembolic events. No increase in venous thromboembolic events was observed with bevacizumab in either study
1. Adapted from Miller et al. N Engl J Med 2007;357-2666-76.2. Adapted from Miles et al. ASCO 2008, abstract LBA 1011.

E2100 Study Design: Randomized, double-blind, placebo-controlled, multicentre, phase III trial
• Primary end point: progression-free survival (PFS)
• Secondary end points: overall response rate, overall survival, quality of life
Previously untreated MBC
(n=722)
Paclitaxel 90 mg/m2 qw for 3 weeks of a
4-week cycle(n=354)
Paclitaxel + bevacizumab10 mg/kg q2w
(n=368)
Progressive disease (PD)*
PD
*No crossover permitted
Adapted from Miller et al. N Engl J Med 2007;357:2666-76.

0.0
0.2
0.4
0.6
0.8
1.0
Months
PF
S p
ropo
rtio
n
0 6 12 18 24 30
E2100 Trial: PFS Results
HR=0.51 (0.43-0.62)
Log rank test P<0.0001
Paclitaxel/bevacizumab: 11.4 months
Paclitaxel: 6.11 months
484 events reported (89% of required events)
6.11 11.4
Adapted from Miller et al. N Engl J Med 2007;357:2666-76.

E2100 Trial: Overall Response Rate
Ove
rall
resp
onse
rat
e (%
)
339 341 262 236
P<0.0001
P<0.0001
Paclitaxel
Paclitaxel/bevacizumab
13.8
29.9
16.0
37.7
0
10
20
30
40
All patients Measurable disease
Adapted from Miller et al. N Engl J Med 2007;357:2666-76.

AVADO Study Design: Randomized, double-blind, placebo-controlled, multicentre, phase III trial
Primary end point: PFS
Secondary end points: overall response rate, duration of response, time to treatment failure, overall survival, safety and quality of life
Previously untreated MBC
(n=705)
Docetaxel 100 mg/m2 q3w+
placeboPD
Docetaxel + bevacizumab7.5 mg/kg q3w
Docetaxel + bevacizumab15 mg/kg q3w
Docetaxel was administered for a maximum of nine cycles but earlier discontinuation was permitted
PD
PD
Adapted from Miles et al. ASCO 2008, abstract LBA1011.
All patients were given the option
to receive bevacizumab
with second line chemotherapy

Adapted from Miles et al. ASCO 2008, abstract LBA1011.
AVADO: Response (patients with measurable disease), %
Placebo+ docetaxel
(n=207)
Bev 7.5†
+ docetaxel (n=201)Bev 15†
+ docetaxel (n=206)
Overall response rate
P value (vs. control)
44
-
55
0.0295
63
0.0001
Best responseCR 1 3 1PR 44 52 62SD 39 35 25
PD 12 5 4
†mg/kg q3w

Adapted from Miles et al. ASCO 2008, abstract LBA1011.
AVADO: Safety Summary
%
Placebo+ docetaxel
(n=233)
Bev 7.5†
+ docetaxel (n=250)
Bev 15†
+ docetaxel (n=247)
Any adverse events (AE) 99.6 100.0 99.6
Any grade ≥3 AE 67.0 74.8 74.1
AEs leading to death* 2.6 1.6 1.6
AEs leading to discontinuation‡ of
Docetaxel 24.0 20.8 24.3
Bevacizumab or placebo 11.2 8.0 11.7
†mg/kg q3w; †during study phase; ‡not mutually exclusive.

Adapted from Miles et al. ASCO 2008, abstract LBA1011.
AVADO: Grade ≥3 Adverse Events of Special Interest,* %
Adverse event, %
Placebo+ docetaxel (n=233)
Bev 7.5† + docetaxel (n=250)
Bev 15† + docetaxel (n=247)
All 31.3 35.2 36.4
Neutropenia 17.2 19.2 19.8
Febrile neutropenia 12.0 15.2 16.6
Venous thromboembolism 3.4 1.2 1.2
Hypertension 1.3 0.4 3.2
Bleeding 0.9 1.2 1.2
Wound-healing complication 0.9 0.4 0.4
GI perforation 0.9 0.4 0.4
Congestive heart failure 0 0.8 0
ATE 0.4 0 0
Proteinuria 0 0 0.4
RPLS** 0 0 0
*Protocol-defined; †mg/kg q3w; **RPLS=reversible posterior leuko encephalopathy syndrome

Ongoing RIBBON 1 Phase IIITrial Study Design
Primary end point: hierarchical PFS
Anthracycline-based combination chemotherapy, Q3w taxane (docetaxel or protein-bound paclitaxel) or capecitabine as determined by investigator prior to randomization † Chemotherapy regimen at investigator discretion
Chemotherapy*+ bevacizumabi.v. 15mg/kg q3w
Chemotherapy* + placebo
(i.v. on day 1 of 21-day cycle)
Previously untreated MBC
(n=950), 2:1 Randomization
PD
PD
Chemotherapy† + bevacizumab i.v. 15mg/kg q3w or 10
mg/kg q2w
Adapted from Albain K. ASCO 2008.
Chemotherapy†
+ crossover to bevacizumab
15mg/kg q3w or 10 mg/kg q2w