Automated Microreactor Optimisation of the Swern-Moffatt...
Transcript of Automated Microreactor Optimisation of the Swern-Moffatt...
Swern-Moffatt oxidation
We investigated the Swern-Moffatt oxidation of benzylalcohol (1) as a selective procedure for the preparation of benzaldehyde (2) in flow. Conventionally, low temperature conditions are required in order to prevent formation of side products 3 and 4. In continuous flow, when short reaction times are employed, significantly higher temperatures can be used.3,4 While DIPEA was used as a base to perform the final step in the reaction, it was also found to be an adequate quenching agent to stop the reaction and ensure well-defined reaction times in the continuous flow system.
Small microreactor channels (1 μm to 1 mm) enable a different way of performing synthetic chemistry: extremely short contact times can circumvent the need for performing highly exothermic and fast reactions at very low temperatures.1 In order to fully exploit this technology, such fast processes need to be redesigned and investigated for optimal reaction conditions, which can differ drastically from the ones traditionally applied. We optimised the selective Swern-Moffatt oxidation of benzyl alcohol to benzaldehyde by varying five experimental parameters in an automated microreactor setup.2
Automated Microreactor Optimisation of the Swern-Moffatt Oxidation at Elevated Temperatures
FutureChemistry’s FlowScreen automated optimisation setup was used.
The system was equipped with glass microreactors with volumes of 0.14 and 7.0 μL in order to cover a wide range of reaction times.
Experimental conditions for continuous flow were determined. Dichloromethane was used as a solvent. Trial runs were performed to ensure robust behaviour of the liquids in the flow system. The ranges of parameters to be optimised were determined in univariate experiments:
Temperature: 25 - 70 °C Reaction time: 0.03 - 3.55 s TFAA stoichiometry: 1.0 - 8.5 DMSO stoichiometry: 2.5 - 10 Alcohol concentration: 0.15 - 0.25 M
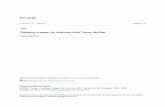
These contour plots represent a selection of a polynomial model fit.
Number of experiments: 180
Optimal parameters: Temperature: 70 °C Reaction time: 0.032 s TFAA stoichiometry: 6 DMSO stoichiometry: 9
Step 4: Lab scale continuous flow
Step 3: Data processing
Step 2: Optimisation in a microreactor system
Step 1: Continuous flow system
Conclusions
The reaction was performed in a larger continuous flow setup in order to synthesise the aldehyde at a preparative scale. Optimal parameters, established in step 3, were used.
Full scale results: Total run time: 120 min Substrate throughput: 0.5 g h-1
Yield based on GC: 96% Isolated yield of aldehyde: 1.70 g
We have applied an automated microreactor platform to optimise a very fast and exothermic reaction. Optimal conditions were found at a very short mixing and reaction time of only 32 ms and a reaction temperature of 70 °C, approximately 150 °C higher than under conventional batch conditions. This remarkable difference shows the potency of continuous flow chemistry. The optimal conditions were also successfully applied to a larger microreactor system at a substrate throughput of 0.5 g h-1, which clearly underlines the potential of flow chemistry in organic synthesis.
[1] Yoshida, J. I.; Nagaki, A.; Yamada, T., Flash chemistry: Fast chemical synthesis by using microreactors. Chem. Eur. J., 2008, 14 (25), 7450-7459.
[2] P.J. Nieuwland, K. Koch, N.v. Harskamp, R. Wehrens, J.C.M.v. Hest and F.P.J.T. Rutjes; Flash chemistry extensively optimized: high temperature Swern-Moffatt oxidation in an automated microreactor platform, Chem-Asian J., 2010, 5 (4), 799-805.
[3] Kawaguchi, T.; Miyata, H.; Ataka, K.; Mae, K.; Yoshida, J., Room-temperature swern oxidations by using a microscale flow system. Angew. Chem. Int. Ed., 2005, 44 (16), 2413-2416.
[4] Van der Linden, J. J. M.; Hilberink, P. W.; Kronenburg, C. M. P.; Kemperman, G. J., Investigation of the Moffatt-Swern Oxidation in a Continuous Flow Micro Reactor System. Org. Process Res. Dev., 2008, 12 (5), 911-920.
Pieter J. Nieuwland1*, Kaspar Koch1, Noud van Harskamp2, Ron Wehrens2, Jan C.M. van Hest2 and Floris P.J.T. Rutjes2
1FutureChemistry Holding BV, Toernooiveld 100, 6525 EC Nijmegen, the Netherlands2Radboud University Nijmegen, Institute for Molecules and Materials, Heyendaalseweg 135, 6525 AJ Nijmegen, the Netherlands
DM
SO S
toic
hiom
etry
0
2.7
5.3
8
Tim
e (s
)
0.032
0.11
0.37
1.3 Ald
ehyd
e yi
eld
(%)
Tem
pera
ture
(°C
)
30
41
51
62
TFAA Stoichiometry0 2.7 5.3 8
0
20
40
60
80
100
Temperature control