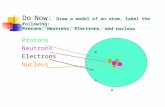
Protons + Neutrons = Nucleus Electrons “orbit” at various distances from nucleus
Atomic Structure Notes. All atoms consist of a small, massive nucleus surrounded by smaller, lighter...
-
Upload
sarah-chambers -
Category
Documents
-
view
212 -
download
0
Transcript of Atomic Structure Notes. All atoms consist of a small, massive nucleus surrounded by smaller, lighter...

Atomic Structure Notes

• All atoms consist of a small, massive nucleus surrounded by smaller, lighter electrons
• The nucleus consists of protons and neutrons
• The electrons are negatively charged, protons are positively charged, neutrons are neutral (zero charge)

Protons (+)
Electrons (-)
Neutrons
BeBeryllium
4
9.012
Protons + Neutrons = Atomic Mass
Chemical Symbol for Element BerylliumA
tom
ic N
umbe
r# of Protons = # of Electrons

• Because atoms are so small they are measured using Atomic Mass Units (AMU)
• Hydrogen is one AMU and is used as a “measuring stick” for all other atoms
• One Proton is about 1 AMU
• One Neutron is about 1 AMU
• It takes about 2000 Electrons to make one AMU

Valence Electrons
• The electrons furthest from the nucleus are called valence electrons.
• These electrons are involved in the formation of chemical bonds.
• A chemical bond forms between atoms when valence electrons are shared or transferred between two or more atoms.

Atoms Are Neutral
• The number of protons(+) and electrons(-) in an atom are equal; the overall atomic charge is zero, therefore the atom is electrically neutral.

Electron Dot Diagram
• Electron Dot Diagrams are used to show the number of valence electrons present in an atom. Here is an atom of Sodium
Na,,,,,

Electron Dot Diagram
• Here is an electron dot diagram for oxygen.
O,,,,,
,,,,,
,,,,,
,,,,,
,,,,,
,,,,,

Electrons in Orbit
• Electrons are held in stable ORBITS (ENERGY SHELLS) around the nucleus by a balance between two opposing forces:

1st Force Holding the Electrons in Orbit
• The force of electrostatic attraction pulls the negative electron inward, towards the positive nucleus.

2nd Force Holding Electrons In Orbit
• The 2nd force is an outward force. It is caused by the accelerating electron known as the CENTRIPETAL FORCE (Same force present when swinging a rock on the end of a string.)

Electron Configuration
• Only two electrons can fit in the first orbit. It is then considered full so remaining electrons need to be placed in the second, third, fourth, etc… orbits.
• Many periodic tables will tell how many electrons are found in each orbit.
• If an atom is in the 1st row of the periodic table (period) it will have one orbit. If an atom is in the second row it will have two orbits. Third row will have three, and so on.

Location in the Periodic Table.
• This atom is found in the second row. It has two orbits.
• It has four red protons so it is Atomic #4 in the periodic table. What is its name?
Use your periodic table to identify.

Practice Drawing
• Draw an atom of Nitrogen and label all of its particles.

How Did You Do?
• Nitrogen is in the second row so it has two orbits surrounding its nucleus.
• The nucleus should contain 7 protons.
• There should be 7 total electrons. 2 in the first orbit and 5 in the second orbit.
• Figure the number of neutrons by subtracting the number of protons from the atomic mass. (14 – 7) = 7 Neutrons.

Questions About Atoms
• Visit the link below and select 5 questions about atoms that you would like the answer to.
• Summarize the question and the answer on the next page of your composition notebook.
• http://education.jlab.org/qa/archive_idx.html