Aplastic Anemia and Monosomy 7–Associated
-
Upload
nadya-erlianie -
Category
Documents
-
view
219 -
download
0
Transcript of Aplastic Anemia and Monosomy 7–Associated
-
8/12/2019 Aplastic Anemia and Monosomy 7Associated
1/6
Am J Clin Pathol 2006;126:925-930 925925 DOI: 10.1309/50GWDKVWU3VWL5XW 925
American Society for Clinical Pathology
Hematopathology / A PLASTIC A NEMIA AND DYSMEGAKARYOCYTOPOIESIS
Aplastic Anemia and Monosomy 7AssociatedDysmegakaryocytopoiesis
Michelle M. Dolan, MD, 1 Timothy P. Singleton, MD, 1 Joseph Neglia, MD, MPH, 2
and Adina Cioc, MD 1
Key Words: Aplastic anemia; Monosomy 7; Myelodysplasia
DOI: 10.1309/50GWDKVWU3VWL5XW
A b s t r a c t
Aplastic anemia (AA) is marrow failure due to aninadequate number of hematopoietic cells in themarrow. Prior reports have described a moreaggressive clinical course in aplastic anemia withmonosomy 7.
We report 3 pediatric cases of AA with normalcytogenetics followed by acquisition of monosomy 7.
Bone marrow biopsies were initially diagnostic of AA
but later showed monosomy 7 and an increased number of megakaryocytes with small hypolobated nuclei.
Immunohistochemical stains for CD61 highlighted themarked dysmegakaryocytopoiesis. The marrow blast
percentage was increased in only 1 patient with 4.6%blasts. The 3 patients underwent bone marrowtransplantation, and each has remained disease free for 7 to 18 months after transplantation.
Pediatric patients with AA and normal cytogeneticsmay develop monosomy 7 with a myelodysplasticsyndrome, unclassified. Patients with AA and
monosomy 7 should be evaluated for dysmegakaryocytopoiesis.
Aplastic anemia (AA) is a bone marrow failure syndromethat is believed to be due to immunologic destruction of other-wise normal hematopoietic cells. 1 Outcome depends on the eti-ology and treatment, which may include antithymocyte globu-lin (ATG), cyclosporine, other immunosuppressive regimens,and bone marrow transplantation (BMT). In a subset of patients,myelodysplastic syndromes (MDSs) or acute leukemias maydevelop. 2-17 The most frequently reported cytogenetic abnor-mality in these cases is monosomy 7. 2-11,13,14 Reports have
described a more aggressive clinical course in AA with mono-somy 7, a cytogenetic abnormality associated with MDS.
We describe 3 pediatric patients with AA in whom mono-somy 7 developed during the course of their disease. Thesepatients received similar therapeutic regimens, includingATG, steroids, and cyclosporine. Two boys, ages 9 and 12years, were previously healthy. A 17-year-old girl had a histo-ry of chronic active hepatitis. Bone marrow biopsies were ini-tially diagnostic of AA and later showed monosomy 7 andmyelodysplasia characterized by an increased number of small hypolobated megakaryocytes, highlighted with
immunohistochemical stains for CD61. Within weeks tomonths of the development of monosomy 7, the 3 patientsunderwent BMT, and each has remained disease free for 7 to18 months after transplantation.
Materials and Methods
Case Definition
We reviewed the hematopathology database of theUniversity of Minnesota Medical Center, Fairview, from January
-
8/12/2019 Aplastic Anemia and Monosomy 7Associated
2/6
926 Am J Clin Pathol 2006;126:925-930926 DOI: 10.1309/50GWDKVWU3VWL5XW
American Society for Clinical Pathology
Dolan et al / A PLASTIC A NEMIA AND DYSMEGAKARYOCYTOPOIESIS
1993 to December 2005 for pediatric patients (age 18 yearsand younger) with AA who had cytogenetic analyses per-formed at this institution. AA was defined as cytopenias dueto an inadequate number of hematopoietic cells in the mar-row. 18 Patients with Fanconi anemia were excluded. Of 42patients with AA, 3 had normal cytogenetics followed byacquisition of monosomy 7. The peripheral blood smears andbone marrow biopsy specimens were evaluated for myelodys-plastic features before and after development of monosomy 7.The medical records of these patients were reviewed for clin-ical manifestations, therapy, and outcome.
Hematopathologic Studies
The peripheral blood smears, bone marrow aspiratesmears, and touch imprints were air dried and stained withWright-Giemsa. Bone marrow aspirate slides were stained withDacie for sideroblastic iron. 19 Bone marrow biopsy specimenswere fixed in B-5 or acetic zinc formalin, decalcified, andembedded in paraffin. Then 3-m sections were cut and stainedwith H&E and periodic acidSchiff. Immunohistochemicalanalysis was performed on the trephine biopsy specimens forCD61 (2F2, Ventana, Tucson, AZ).
Cytogenetic Analysis
Cells from the bone marrow samples were cultured for 24to 48 hours according to routine cytogenetic protocols.Chromosome analysis was performed on metaphases G-band-ed with trypsin and Wright-Giemsa stain. Twenty metaphasecells were analyzed for each patient.
Fluorescence In Situ Hybridization Analysis
Fluorescence in situ hybridization (FISH) was performedusing a dual-color probe set to the elastin gene region in band7q11.23 and loci D7S486 and D7S522 in band 7q31 or a dual-color probe set to D7Z1 and EGFR, mapped to the centromereregion of chromosome 7 and to 7p12, respectively (Vysis, DesPlaines, IL). Use of a dual-color probe set decreases the prob-ability of false-positive results, as loss of 1 signal from eachlocus was required for designation as a monosomy 7 cell.Reference ranges for a monosomy 7 signal pattern in normal
control samples is 0% to 1%. FISH was performed accordingto the manufacturers instructions. We examined 200 to 400interphase cells with these probes.
Results
Clinical Histories
Case 1
A previously healthy 11-year-old boy was diagnosed withAA and was treated intermittently with steroids, cyclosporine,and ATG for 19 months. Although the results of cytogenetic
analysis remained normal during therapy, monosomy 7 devel-oped shortly after cessation of immunosuppressive therapy.He then underwent allogeneic BMT and remained disease-free 11 months later.
Case 2A previously healthy 12-year-old boy was diagnosed with
AA. For approximately 3 years of therapy with steroids,cyclosporine, and ATG, his cytogenetic analyses showed noclonal abnormality. Approximately 34 months after diagnosis,monosomy 7 was detected. At 42 months after diagnosis, thepatient underwent matched unrelated-donor BMT. His subse-quent clinical course was complicated by the development of a posttransplantation lymphoproliferative disorder 9 monthsafter BMT. Examination of this tissue showed no evidence of monosomy 7.
Case 3A 17-year-old girl was hospitalized for hepatitis of
unknown origin; liver biopsy showed cholestatic hepatitis.One month later, she was hospitalized with headaches, easybruising, and fatigue. She was diagnosed with AA. She initial-ly received supportive therapy (packed RBCs and platelettransfusions and antibiotic prophylaxis), but 2 months laterreceived cyclosporine, high-dose steroids, and granulocytecolony-stimulating factor (G-CSF). Her clinical course wascomplicated by herpetic mouth sores and an Escherichia coliurinary tract infection and bacteremia and by increased trans-fusion needs. Cytogenetic analysis results remained normal.
However, 15 months after the diagnosis of AA, her bone mar-row showed monosomy 7. Approximately 2 weeks later, sheunderwent an unrelated umbilical cord blood transplant andremained disease-free 1 year later.
Hematopathologic and Cytogenetic Findings
The blood cell counts, bone marrow cellularity, and mor-phologic dysplasia at presentation and at diagnosis of mono-somy 7 are summarized in Table 1 . Table 2 lists the cyto-genetic findings at various times after diagnosis of AA.
Case 1At presentation with AA, the marrow showed 30% cellu-
larity with decreased trilineage hematopoietic cells and slightdyserythropoiesis, including rare binucleated cells, slightmegaloblastoid changes, and rare nuclei with irregular con-tours. The marrow cellularity varied between 10% and 60% onsubsequent evaluations. The results of cytogenetic analyses of 5 bone marrow aspirate specimens were normal. Nineteenmonths after diagnosis, the bone marrow had cellularity of 70% to 80%, slight dyserythropoiesis, and marked dys-megakaryocytopoiesis with numerous dysplastic megakaryo-cytes with small hypolobated nuclei or separate round nuclear
-
8/12/2019 Aplastic Anemia and Monosomy 7Associated
3/6
Am J Clin Pathol 2006;126:925-930 927927 DOI: 10.1309/50GWDKVWU3VWL5XW 927
American Society for Clinical Pathology
Hematopathology / O RIGINAL ARTICLE
Table 1 Blood and Bone Marrow Findings in AA and at Acquisition of Monosomy 7 *
Case No./ WBC Plt Marrow MarrowDiagnosis Hgb (g/L) (10 9 /L) (10 9 /L) Cellularity (%) Morphologic Features G-Bands FISH (%)
1AA 91 8.4 106 30 Slight dyserythropoiesis 46,XY (100%) NAMDS 113 3.1 108 70-80 Slight dyserythropoiesis; dysmegakaryo- 45,XY,7 (75%) 29
cytopoiesis;
-
8/12/2019 Aplastic Anemia and Monosomy 7Associated
4/6
928 Am J Clin Pathol 2006;126:925-930928 DOI: 10.1309/50GWDKVWU3VWL5XW
American Society for Clinical Pathology
Dolan et al / A PLASTIC A NEMIA AND DYSMEGAKARYOCYTOPOIESIS
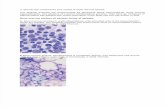
segments Image 1 . Immunohistochemical stains for CD61showed numerous small dysplastic megakaryocytes Image 2 .These bone marrow biopsy findings were consistent with anMDS, unclassified. G-banded cytogenetic analysis showed60% of metaphase cells with monosomy 7. In a follow-up bonemarrow specimen obtained 3 weeks later, 75% of G-bandedmetaphase cells had monosomy 7 and 29% of interphase cellshad a monosomy 7 signal pattern by FISH (Table 2).
Case 2The diagnostic bone marrow biopsy showed AA with 30%
to 40% cellularity, decreased trilineage hematopoietic cells, andslight dyserythropoiesis, including slight megaloblastoid
changes, rare internuclear bridges, rare nuclear buds, and rarenuclei with irregular contours. Three cytogenetic analyses per-formed at different times were karyotypically normal.Approximately 3 years after diagnosis, cytogenetic analysisshowed 50% of the G-banded metaphase cells to have mono-somy 7. The bone marrow biopsy showed 30% cellularity andslight to moderate dyserythropoiesis, including rare internu-clear bridges, rare nuclear buds, and rare nuclei with irregularcontours. The aspirate smears and trephine sections hadonly rare megakaryocytes detected with routine stains, andthese often had small hypolobated nuclei. Stains for CD61 onthe trephine biopsy were not available. Eight months later,cytogenetic studies showed monosomy 7 in 15% to 30% of
A B
Image 2 A , CD61 stain of marrow trephine biopsy highlights numerous dysplastic megakaryocytes after acquisition ofmonosomy 7 (400). B , CD61 stain of marrow trephine biopsy in a prior aplastic anemia with normal karyotype (400).
A B
Image 1 A , Dysmegakaryocytopoiesis after acquisition of monosomy 7 in a marrow aspirate smear (Wright-Giemsa, 2,000).B , Dysmegakaryocytopoiesis after acquisition of monosomy 7 in a marrow trephine biopsy (H&E, 1,200).
-
8/12/2019 Aplastic Anemia and Monosomy 7Associated
5/6
Am J Clin Pathol 2006;126:925-930 929929 DOI: 10.1309/50GWDKVWU3VWL5XW 929
American Society for Clinical Pathology
G-banded metaphase cells and in 18% to 38% of interphasecells by FISH. A bone marrow biopsy showed 25% cellulari-ty, 4.6% blasts, slight dyserythropoiesis, slight dysgranu-lopoiesis (rare hypogranular neutrophils and precursors), andmarked dysmegakaryocytopoiesis with numerous megakaryo-cytes with small hypolobated nuclei and occasionalmicromegakaryocytes, consistent with an MDS, unclassified.CD61 highlighted the dysmegakaryocytopoiesis, includingsmall megakaryocytes that were not readily detected with rou-tine stains.
Case 3At presentation with AA, the bone marrow cellularity was
less than 5% with decreased trilineage hematopoietic cells.Cytogenetic analysis was normal. At 15 months after diagno-sis, the bone marrow had 40% cellularity, slight dysgranu-lopoiesis, and marked dysmegakaryocytopoiesis with numer-ous dysplastic megakaryocytes with small hypolobated nuclei,consistent with an MDS, unclassified. Cytogenetic studiesshowed 25% of G-banded metaphase cells to have monosomy7; FISH showed 7.8% of interphase cells with only 1 chromo-some 7 signal.
Discussion
AA is a bone marrow failure syndrome of adults and chil-dren that, most evidence suggests, is due to immune-mediatedsuppression of otherwise normal hematopoietic cells. 1 First-
line therapy of this disorder has consisted largely of immuno-suppressive regimens including cyclosporine, ATG, andsteroids with or without the addition of G-CSF. 1 Althoughsome studies have shown that AA is a polyclonal disorder,consistent with the theory that AA is an autoimmune responsedirected against hematopoietic stem cells, 1 approximately13% of patients show clonality via molecular testing of X-chromosome markers. 20 Clonal chromosomal abnormalitieshave been reported in only 4% to 10% of marrow sampleswith AA; these clones may be transient or persistent, and arethe types also seen in MDS and acute myeloid
leukemia.15,16,21
More than half of all pediatric AA cases withclonal abnormalities at diagnosis respond well to the first trialof immunosuppressive therapy, 22 but AA and MDS mayrespond to immunosuppressive therapy. 2,23
The distinction between AA and hypocellular MDS isdifficult. Morphologic evaluations are hampered by a paucityof cells owing to sparse hematopoiesis in patchy foci. BothAA and MDS may have dyserythropoiesis. Circulatingmicromegakaryocytes, circulating blasts, ringed sideroblasts,dysgranulopoiesis, dysmegakaryocytopoiesis, abnormal local-ization of immature precursors, marrow fibrosis, andincreased blasts would be more consistent with MDS. 2,23-26
These morphologic features also may be acquired in evolutionfrom AA to MDS. In the present study, 3 cases of AA with nor-mal cytogenetics developed MDS with dysmegakaryocy-topoiesis and monosomy 7. A previous study suggested thatstaining of marrow trephine biopsy specimens for CD34 andproliferating cell nuclear antigen may be helpful in the differen-tial diagnosis of AA vs MDS. 27 The present study proposesstaining for CD61 to evaluate megakaryocytes. Although mono-somy 7 has been shown to involve megakaryocytes, 28 it is notspecific for the megakaryocytic lineage in myeloid disorders. 29,30
A number of studies have shown that clonal progressionof AA occurs during or after immunosuppressive therapy withor without G-CSF; in 5% to 15% of all long-term survivors of AA, MDS or AML develops. 17 Among cases of AA thatprogress to MDS or acute myeloid leukemia, monosomy 7 isthe most commonly reported cytogenetic abnormality. 3,16,21,22
We describe 3 patients with AA and normal cytogeneticstudies in whom monosomy 7 with an MDS, unclassified,characterized by marked dysmegakaryocytopoiesis laterdeveloped. Dysmegakaryocytopoiesis may be difficult toassess in hypocellular aspirates and trephine biopsy speci-mens but can be highlighted with stains for CD61 on themarrow trephine biopsy specimen. Given that AA withmonosomy 7 is reported to have a prognosis similar toMDS, 3 our study suggests that cases of AA with monosomy7 should be evaluated carefully for dysmegakaryocy-topoiesis that would indicate an MDS.
From the Departments of 1 Laboratory Medicine and Pathology
and2
Pediatric Hematology-Oncology, University of Minnesota Medical School, Minneapolis.
Address reprint requests to Michelle Dolan: Dept of Laboratory Medicine and Pathology, University of Minnesota Medical School, Mayo Mail Code 609, 420 Delaware St SE, Minneapolis, MN 55455.
Acknowledgment: We thank Betsy Hirsch, PhD, for helpfuldiscussions regarding this project.
References1. Young NS, Maciejewski J. The pathophysiology of acquired
aplastic anemia. N Engl J Med. 1997;336:1365-1372.2. Maciejewski J, Selleri C. Evolution of clonal cytogenetic
abnormalities in aplastic anemia. Leuk Lymphoma.2004;45:433-440.
3. Maciejewski JP, Risitano A, Sloand EM, et al. Distinct clinicaloutcomes for cytogenetic abnormalities evolving from aplasticanemia. Blood. 2002;99:3129-3135.
4. Kojima S, Ohara A, Tsuchida M, et al. Risk factors forevolution of acquired aplastic anemia into myelodysplasticsyndrome and acute myeloid leukemia after immunosuppressivetherapy in children. Blood.2002;100:786-790.
5. Kaito K, Kobayashi M, Katayama T, et al. Long-termadministration of G-CSF for aplastic anemia is closely relatedto the early evolution of monosomy 7 MDS in adults. Br JHaematol. 1998;103:297-303.
Hematopathology / O RIGINAL ARTICLE
-
8/12/2019 Aplastic Anemia and Monosomy 7Associated
6/6