Apical targeting in polarized epithelial...was observed in the Golgi^^, the possibility that the...
Transcript of Apical targeting in polarized epithelial...was observed in the Golgi^^, the possibility that the...

One particularly well-studied example of polarizedcells is epithelial cells, which form a layer lining asurface or cavity. Of the approximately 160 recognized major cell types in humans, around 60% areclassified as epithelial. The plasma membrane of epithelial cells is divided by tight junctions into twodomains: an apical surface facing a lumen or the outside world, and a basolateral surface facing adjoiningcells and underlying connective tissue. These two domains have different protein and lipid compositions,reflecting their very different functions. To appreciatethe importance of this polarity, imagine the consequences if the epithelia of the exocrine pancreas orthe stomach suddenly secreted digestive enzymes orhydrochloric acid, respectively, to the 'other side'.
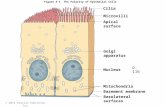
Epithelial cells use two pathways to send moleculesto the correct surface (Fig. 1). In the 'direct' pathway, new protein molecules are synthesized in therough endoplasmic reticulum (RER) and transportedthrough the Golgi to the trans Golgi network (TGN).In the TGN, proteins are packaged into vesicles thatdeliver them to the apical or basolateral surface.Alternatively, in the 'indirect' pathway, moleculesare sent first from the TGN to the basolateral surface,from which they can then be endocytosed and transported to the apical surface by transcytosis. Trans-cytosis (at least to the apical surface) involves transit through an apical recycling compartment (ARC),which is a central sorting station in the endosomalsystem ^ The steady-state distribution of a protein candepend not only on its vectorial delivery to each location but also on its retention, for example by binding to the membrane cytoskeleton as proposed forthe Na+/K"^-ATPase2. We divide polarized transportto the plasma membrane into four steps: segregation,budding, transport and docking (Fig. 1). Here, weconcentrate primarily on the first and last steps.
Step 1: segregationAt some point in both the direct and indirect path
ways, apical and basolateral proteins must be sepa r a t e d f r o m e a c h o t h e r w h i l e s t i l l i n t h e s a m e m e mbrane. This sorting information must be an intrinsicproperty of the proteins, although later sorting stepsmight depend solely on the properties of the vesicle(or the raft: see below) containing the proteins.
Sorting information for basolateral membrane proteins is usually encoded in short (2-10 residue) 'basolateral sorting signals' located in the cytoplasmic domain of a proteinT These frequently resemble or evenoverlap with Tyr-containing or Leu-Leu motifs usedfor endocytosis from the plasma membrane or forsorting from the TGN to endosomes. However, mutagenesis studies demonstrate distinct sequence requirements for basolateral sorting and endocytosis.A revealing example is the basolateral sorting signalof the polymeric immunoglobulin receptor (plgR)'^.This signal lacks a Tyr or Leu-Leu motif, but its secondary structure includes a crucial type I p-turn, likethat found in Tyr-containing endocytosis signals. Itis possible that the fundamental feature of all ofthese sorting signals is a type I p-turn.
The best-understood apical signal is the glycosyl-phosphatidylinositol (GPI) anchor, and its properties
Apical targeting inpolarized epithelialce l l s : t he re ' s more
afloat than ra f ts
Most metazoan cells are 'polarized'. A crucial aspect of this
polarization is that the plasma membrane is divided into two ormore domains with different protein and lipid compositions -
for example, the apical and basolateral domains of epithelial cellsor the axonal and somatodendritic domains of neurons. This
polarity is established and maintained by highly specific vesicularmembrane transport in the biosynthetic, endocytic and transcytotic
pathways. Two important concepts, the 'SNARE' and the 'raft'
hypotheses, have been developed that together promise at least a
partial understanding of the underlying general mechanisms thatensure the necessary specificity of these pathways.
have led to the emergence of a general model for sorting of apically targeted proteins and lipids^. A centralfeature of the model is clustering of glycosphingo-lipids (GSLs) and GPl-anchored proteins (GPIAPs) intod i s t i n c t m e m b r a n e s u b d o m a i n s o r ' r a f t s ' . I n a r t i fi c i a l -
membrane models, GSLs spontaneously self associateinto such rafts, possibly through hydrogen bondingof their head groups and/or packing of their long,saturated acyl chains into a 'liquid ordered' phase^.Cholesterol promotes formation of this phase, perhaps by intercalating between the acyl chains of theGSL^'^ The outer leaflet of the apical plasma membrane of a typical epithelial cell is enriched in certainglycosphingolipids and depleted of glycerolipids.GPIAPs are found predominantly at the apical surfaceof most epithelial cells, and the addition of a GPIanchor is sufficient to target a reporter protein to theapical surface. In the 'raft hypothesis', GPIAPs andGSLs meet in the biosynthetic pathway and clustertogether spontaneously to form a membrane micro-domain, or raft. The association of GPIAPs with raftsmay be due to the long acyl chains in the GPI anchor.Raft formation is the sorting process perse, and the intact raft is transported in vesicles to the apical surface.
Biophysical techniques have been used to demonstrate the existence of rafts, primarily in artificialmembranes^. However, for studies in cells, the main
The authors are in
the Dept ofAnatomy, Dept ofBiochemistry andBiophysics, andC a r d i o v a s c u l a r
Research Institute,University ofCalifornia,San Francisco,
CA 94143-0452,USA.
T. W. and S. H. L
are currently at:M e d i z i n i s c h e
trends in CELL BIOLOGY (Vol. 7) October 1997 Copyright © 1997 Elsevier Science Ltd. All rights reserved. 0962-8924/97/$17.00 393Pll: S0962.8924(97)01130-6

rev iews
D i r e c t p a t h w a y T r a n s c y t o s i sF I G U R E 1
Summary of the vesicular membrane-trafficking pathways in a typical epithelial ceil (e.g. MDCK). Epithelial cells possess two plasmamembrane domains, apical and basolateral, separated by tight junctions (T)s). Two principal pathways exist for the targeting of
plasma membrane proteins: in the 'direct' pathway, proteins are sorted in the Golgi apparatus, possibly by clustering into or exclusionfrom glycosphingolipid-rich membrane microdomains (rafts, step 1). Transport vesicles destined for the apical and basolateral
membranes bud from the trans Golgi network (TCN), in a process probably mediated by coat proteins (step 2). Vesicles aretransported directionally along microtubules (MTs) or other cytoskeletal elements using vesicle-associated motors (step 3).
After reaching the plasma membrane, vesicles dock and fuse utilizing the SNARE machinery at the basolateral and possibly also at theapical surface (step 4, see text for explanations). In the 'indirect' pathway, newly synthesized membrane proteins are first transportedfrom the TGN to the basolateral surface and are then endocytosed into basolateral early endosomes (BEE). From here, apical proteins
are transported along microtubules to the tubovesicular 'apical recycling compartment' (ARC), which also receives proteinsinternalized from the apical surface. The final transport step to the apical plasma membrane Involves the SNARE machinery since it is
NSF-dependent and sensitive to botulinum toxin E (BotTx-E), which cleaves certain t-SNAREs^®.
experimental tool used to determine whether a molecule partitions into rafts, and indeed part of theunderpinnings of the raft hypothesis, is to soluhilizethe cells in non-ionic detergents (e.g. Triton X-100)at 4°C. Under these conditions, GSLs and GPIAPs arefound in low-density insoluble membrane structuresthat can be isolated on sucrose gradients. Althoughcopurification with such floating material is oftentaken as evidence that a molecule is part of a raft, this,perse, is a poor indication that such a molecule is inr a f t s o r e v e n t h a t t h e r a f t s t h e m s e l v e s e x i s t i n i n t a c tcells"''. For instance, sphingomyelin is almost entirelydetergent insoluble^", yet it, as well as other sphingo-lipids such as galactosylceramide and sulfatide, ispreferentially transported basolaterally in Madin-Darby canine kidney (MDCK) epithelial cells"''^.Similarly, several non-apical proteins are found in
the detergent-insoluble material. The choice of detergent also has a strong influence on the protein andlipid composition of the isolated final material'".
Besides GPI-anchored proteins, some apical transmembrane proteins are also raft associated as judgedby the detergent-insolubility criterion; these includeinfluenza haemagglutinin (HA) and neuraminidase( N A ) T h e t r a n s m e m b r a n e d o m a i n o f N A w a sshown to be responsible for apical targeting as wellas for detergent insolubility", but this appears not tobe the case for HA"'. These findings led to an extension of the raft hypothesis as a general mechanism ofprotein sorting in the biosynthetic pathway. Raft formation and hence sorting may take place as early asin the cis or medial Golgi'". Although this appears tocontradict earlier studies in which no segregation ofapically and basolaterally targeted membrane proteins
3 9 4 trends in CELL BIOLOGY (Vol. 7) October 1997

was observed in the Golgi^^, the possibility that the resistance, sorts GSLs correctly to the apical surface,sorting power of the entire Golgi apparatus could be but GPIAPs are sorted to both surfaces '̂̂ .used for this process is intr iguing. Fischer rat thyroid (FRT) cells sort GSLs and
N-linked oligosaccharides can act as an apical sig- GPIAPs entirely to the basolateral surface^ '̂̂ s. severalnal for secretory proteins, for example gp80, in MDCK other non-GPI-anchored proteins that are apical incells^®-2°. Some basolateral membrane proteins ap- MDCK cells are also apical in these cells, indicatingpear to possess a recessive apical signal in their lu- that FRT cells can still sort proteins to the apical sur-menal domain because removal of their cytoplasmic face in the absence of apical GSL- and GPlAP-traffic.basolateral targeting signal often results in apical tar- HT-29 cells can be grown under conditions where thegeting^L It has been suggested that this signal is the cells are non-polarized and the 'apical membrane'N-glycans^L Moreover, it has been hypothesized that forms an intracellular compartment. It was reportedN-glycans might interact with a raft-bound lectin, that GPIAPs are transported to this 'apical' compart-such as V1P36, in the TGN, thereby accomplishing ment, whereas some normally apical transmembranesorting^L This latter conjecture is, however, incom- proteins are still transported to the surface^^.patible with the finding that certain apical proteins. There are several other shortcomings in the experi-including gp80, can be transported independently mental support for the raft model. First, much of theof GPIAPs and GSLs (Refs 22 and 23 and see below). work on sphingolipid sorting has been based on using
Although the raft model is extremely appealing, it soluble, short chain, fluorescent sphingolipids, suchis useful to examine how well it explains sorting in as NBD ceramide and its metabolites. Although thesevarious epithelial cell types (Table 1). The most com- analogues can be a useful tool for analysing lipid trans-monly used epithelial cell line is MDCK. The original port, they have a bulky artificial fluorescent groupline was heterogeneous, and different clones with and lack the long acyl chain that may be involved indistinctive properties have been isolated by several packaging into rafts. Second, it has been realized regroups. In general, t5^e 1 clones have high transmono- cently that these short-chain lipid analogues can belayer electrical resistance (-1000 ohm cm-^), whereas transported independently of vesicular traffic owingtype 11 clones have lower resistance (-100 ohm cm-^). to their high water-solubility^^, requiring a revision ofOne of the most frequently used MDCK type 11 clones the interpretation of many previously published data,was isolated at the EMBL in Heidelberg ('Heidelberg Third, little attention has been paid to variations in theclone'). Remarkably, another type 11 clone ('J clone') length and saturation of the acyl groups in varioussorts GSLs and GPIAPs equally to both surfaces, lipids. Such variations could account for at least somewhereas both a transmembrane apical protein (gpl35) of the differences in sorting seen in different cell types.and a secretory apical protein (gp80) are still sorted Similarly, the structure of the GPl anchors may varyto the apical surface^^. This cell line can therefore in different proteins and cell types, which might alsosort certain proteins to the apical surface independ- account for some of the differences described above,ently of GSLs and GPIAPs. Another MDCK t̂ e 11 We therefore suggest that, although the raft modelstrain, isolated by selection for concanavalin A is appealing, it is far from well established and should
TABLE 1 - SUMMARY OF SORTING PATTERNS FOUND IN A VARIETY OF EPITHELIAL CELLS«
Ceil type Glycolipids GPI-p r o t e l n s
Other apicalp r o t e i n s
T r i t o n -
i n s o l u b l e
Basolateral prote ins
M D C K l i s t r a i n s
Heidelberg strain'1' strain
ConA^ mutantFischer rat thyroid
(FRT) cellsCaco2 intestinal cells
Hepatocytes
ApicalBasolateral
M i x e dBasolateral
A p i c a l G S L , G P l , H AApical (secreted gp80. Not reported
membrane gpl 35)
A p i c a l G S L , G P l
A p i c a l A p i c a l ( s o m epredominantlybasolateral, thentranscytosed apical)
Baso la tera l Baso la tera l
(followed by transcytosis in both cases)
GSL, GPlGSL, not GPl
GSL, GPl
Mixed (NaVK^-ATPase)Basolateral (E-cadherin)Basolateral
Basolateral
Basolateral
Not reported Basolateral
®These data indicate that there is tremendous plasticity in the sorting patterns used by various epithelial cells. In particular,the notion that glycolipid and GPI-anchored proteins are sorted in Triton-insoluble rafts to the apical surface was establishedin the Heidelberg strain of MDCK cells. Clearly, this pattern does not extend even to other strains of MDCK cells, much lessto other epithelial cell types.Abbreviations: ConA^ concanavalin A resistant; GPl; glycosylphosphatidylinositol; GSL, glycosphingolipid; HA, haemagglu-tinin; MDCK, Madin-Darby canine kidney.
trends in CELL BIOLOGY (Vol. 7) October 1997 3 9 5

not be taken as dogma. Rafts clearly can exist in artificial membranes, and they probably also exist incells. At present, we have no firm idea of the size,localization or dynamic properties of rafts in livingcells. The enormous complexity of lipids and proteinsin real cells may substantially alter the properties ofcellular rafts, compared with those formed in artificial membranes. For instance, caveolin binds to cholesterol and is found in cholesterol-rich caveolae,which may be a specialized type of stabilized, morphologically discernible raft^^. In another example,annexin II associates peripherally with the cytoplasmic surface of cholesterol-rich membranes, and maytherefore interact with rafts, and perhaps link themto the cytoskeleton^^. If rafts do exist in cells, muchwork is required to establish their physiological relevance in protein sorting and other processes.
Some epithelial cells rely on transcytosis for delivery of most of their apical surface components. As FRTcells develop polarity, transcytosis is initially usedfor apical delivery, whereas, later in development,the direct TGN-to-apical route predominates^®. Thus,transcytosis may be more fundamental in ontogenyand even in evolution. In intestinal cells, proteinsuse a mixture of the direct TGN and transcytoticpathways to the apical surface, with the exact percentage for each pathway depending on the individual protein^i'^^. Hepatocytes use transcytosis nearlyexclusively for apical delivery of proteins, evenGPIAPs^^, but they can deliver GSLs directly fromthe TGN to the apical surface^^.
Is the raft mechanism used for sorting during transcytosis? Earlier evidence did not detect GSL sortingand presumably rafting during transc)d:osis in MDCKcells^s. Recently, however, GSL sorting was observedduring transcytosis in hepatocytes^^. How non-GPI-anchored proteins that are not incorporated intorafts transcytose to the apical surface remains animportant question. Perhaps the oligosaccharideson transcytosing proteins interact with a lectin thatis targeted to the apical surface by rafts or anothermechanism. This model might explain how bindingof heavily glycosylated IgA to the pIgR stimulatesapical transcytosis of the pIgRL
Even 'non-polarized fibroblasts', such as babyhamster kidney (BHK) and Chinese hamster ovary(CHO) cells, produce two types of TGN-derived vesicles, corresponding to the apical and basolateralvesicles leaving the TGN in polarized cells^ '̂̂ ®. Thesecells therefore have separate TGN-to-apical andTGN-to-basolateral pathways, including rafts for theapical pathway, but they do not provide separateapical and basolateral plasma membrane targets asthese are mixed in one undifferentiated plasma membrane. However, many fibroblastic cell lines, including the BHK and CHO cells used in these studies, arederived from epithelia and may have only partiallylost epithelial polarity. More surprising is that osteoclasts, which are of non-epithelial, haematopoieticlineage, also have apical and basolateral surfaces aswell as transcytosis^^. The principle of 'apical' and'basolateral' pathways may even hold for Saccharo-myces cerevisiae, which has two pathways from theTGN to the cell surface^®. Since yeast have GPIAPs,
one of these pathways may be the equivalent of theraft pathway. By extension of this idea, the divisioninto apical (GSL raft) and basolateral (cytoplasmicsignal) circuits has been proposed to exist throughout the exocytic and endoc5^ic pathways in bothpolarized and non-polarized cells^. In this model,the apical and basolateral pathways would meet inseveral intracellular compartments, and each compartment would resegregate components into apicala n d b a s o l a t e r a l v e s i c l e s .
Step 2: vesicle formationOnce apical and basolateral proteins have been
sorted from each other, they must be packaged intovesicles that transport them to the respective surfaces. The process of recruitment into a vesicle may infact also contribute to sorting of certain membraneor soluble proteins (in which case, steps 1 and 2would overlap). This would be analogous to the recruitment of receptors and ligands into clathrin-coated pits at the plasma membrane. Although thecompartment from which the final transport vesiclesbud off is generally assumed to be the TGN, somenewly synthesized plasma membrane proteins mightfirst travel from the TGN to endosomes before reaching the plasma membrane'*^''*^. Also, TGN-to-surfacetransport might involve an intermediate(s) that islarger than a classical carrier vesicle, such as a tubule.
How vesicles destined for the apical membrane(including rafts) bud off the TGN is unknown; eventhe involvement of a coat protein is conjectural. Forbasolateral proteins, sorting in the membrane (step 1)and recruitment into budding vesicles (step 2) mayboth involve a coat protein; the similarity of basolateral and endocytosis signals suggests that thiscoat might be a member of the clathrin adaptor andCOP family of coat proteins'^^''^^. A novel adaptorlike complex, termed AP3, has recently been de-scribed'^^'^^. Like the |xl and |x2 subunits of the APIand AP2 adaptor complexes, the |x3 subunit of theAP3 adaptor complex can bind to tyrosine-basedsorting signals. It is possible therefore that this AP3adaptor could be involved in recognition of signalsinvolved in polarized sorting, although similar rolesfor API, as well as other novel adaptors, remain tobe investigated. Myosin II appears to be involved inbudding of basolateral vesicles (Ref. 47, but see alsoRef. 48). It might provide the force for budding orotherwise act on the Golgi spectrin cytoskeleton'^^.Regardless of how sorting and budding occur, a newvesicle must contain information that specifies itstranslocation, docking and fusion properties.
Step 3: vesicle transport to the plasma membraneThe polarized organization of microtubules (MTs)
in epithelia^® suggests that dynein- and kinesin-likemotors could be used for delivery of TGN-derivedvesicles to the apical and basolateral domains, respectively. Indeed, differential requirements for thesemotors have been demonstrated in polarized deliveryin MDCK cells^L Actin-based motors are also likelyto play a role, at least in apical delivery in both thedirect^2 ^nd transcytotic pathways^^. Disruption ofMTs by nocodazole has a kinetic effect on delivery
3 9 6 trends in CELL BIOLOGY (Vol. 7) October 1997

Step 4: docking and fusion of transport vesicieswith the piasma membrane
Once the transport vesicles reach their destination;they must dock to and fuse with the plasma membrane. The SNARE hypothesis provides a unifiedmodel for intracellular membrane fusion^^. This hypothesis postulates that 'addressing' proteins, calledSNAREs (see Box 1), determine the specificity ofmembrane fusion by requiring the correct pairing ofa v-SNARE on the vesicle membrane with its cognatet-SNARE on the target membrane. When a vesiclecarrying digestive enzymes happens to be mistargetedby a failure of prior specificity mechanisms, the pancreas cell has one last chance to prevent the secretion of digestive enzymes into the interstitium: it can
of proteins to both surfaces. From the handful ofproteins examined, it appears that direct apical andtranscytotic delivery are particularly affected, withmany proteins being missorted to some degree tothe basolateral surface. There are two interpretationsfor this disparity. First, transport of apical vesiclesfrom the TGN or basolateral endosomes may relymore on MTs than basolaterally directed pathways;and, in the absence of MTs, these vesicles are relatively free to fuse with either plasma membrane.Such random fusion properties of vesicles does notagree well with either the annexin or SNARE mechanisms of fusion (see below). Second, TGN/endosomesorting of apical proteins could rely more on intactMTs than does that of basolateral proteins, and, inthe absence of MTs, apical proteins are incorporatedinto basolateral vesicles. Sorting in the TGN maybe closely coupled to tubulation^'*. The role of MTs,if any, in this process is unknown, but there ise v i d e n c e f o r M T m o t o r i n v o l v e m e n t i n t u b u l a t i o nof many organelles, including the TGN and endosomes^®. Thus, while MTs are clearly involved incellular organization and vesicle transport in polarized epithelia, their role in targeting specificity isn o t u n d e r s t o o d .
prohibit docking and fusion. The SNARE mechanismmight provide such a final proofreading mechanism.
Recently, however, Ikonen etal. reported that, whiletargeting from the TGN to the basolateral membraneinvolved SNAREs, apical targeting from the TGN wasnot inhibited by antibodies to the general SNARE-dependent fusion factor, NSF, and was insensitive totetanus toxin, which cleaves several v-SNAREs^^. Itwas suggested therefore that TGN-to-apical fusionuses a novel, non-SNARE-dependent pathway andthat the apical surface might even utilize this novelmechanism exclusively. Hence, mistargeted vesiclescould never fuse with the 'wrong' membrane becauseof a complete incompatibility of the machineries.This group found that apically targeted vesicles contain the epithelium-specific annexin 13b, and thatb i v a l e n t a n t i b o d i e s t o a n n e x i n 1 3 b b l o c k e d T G N - t o -
apical delivery, which was interpreted to suggest thatannexin 13b is involved in apical membrane fusion^^
Recently, a similar experimental system was usedto test the role of SNAREs in transcytosis^®. The results showed that both receptor-mediated transcyto-sis of IgA to the apical surface as well as recycling tothe basolateral surface required NSF and were inhibited by botulinum E toxin, which cleaves the neuron-specific t-SNARE SNAP-25. Although it is not clear whatthe target of this toxin is in MDCK cells, new homo-logues of SNAP-25 have been discovered recently^ '̂̂ ®that are good candidates. Thus it appears that the apical plasma membrane domain can utilize the SNAREs,although possibly only for a subset of vesicles.
If SNAREs control the specificity of apical andbasolateral docking/fusion, these domains shouldc o n t a i n d i f f e r e n t t - S N A R E i s o f o r m s . T h i s w a s s h o w n
recently for the t-SNARE subunits of the syntaxinfamily in MDGK^S pancreatic acinar^^ and gastricparietal cells^^ (pjg 2). Syntaxins 2, 3 and 4 are expressed in MDCK cells, but have strikingly differentlocalizations. Syntaxin 2 was found on both theapical and basolateral surfaces, whereas syntaxins 3and 4 localize non-overlappingly to the apical and
BOX 1 - GLOSSARY
M i s c e l l a n e o u sGPIAP; glycosylphosphatidyllnositol (GPI)-anchored protein.GSL: glycosphingolipid.Raft: a membrane microdomain that forms by clustering ofGSLs and GPIAPs.
TGN: trans Golgi network, a proposed major cellular sortingorganelle.
Cell lines (all are of epithelial origin)Caco2 and HT-29 cells: human cell lines derived from colon
c a r c i n o m a .
FRT cells: Fischer rat thyroid cells.MDCK: Madin-Darby canine kidney cells. Different subclones
have been isolated:MDCK I: high transmonolayer electrical resistance.MDCK II: low transmonolayer electrical resistance. Two sub
clones of MDCK II cells have been characterized exten
sively: 'Heidelberg clone' and 'j clone'.
SNARE machineryNSF: N-ethylmaleimide sensitive factor; a soluble cytoplasmic
AT P a s e .
SNAP: soluble NSF attachment protein; recruits NSF to membranes after SNAP binds to a SNARE.
SNAP-25: Synaptosomal-associated protein of 25 kDa: neuron-
specific founding member of the second protein familyacting as t-SNAREs when bound to a member of thesyntaxin family. Unrelated to SNAP.
SNAP-23: ubiquitously expressed homologue of SNAP-25.SNARE: SNAP receptor; membrane proteins on the target
membrane (t-SNARE) or on the vesicle membrane (v-SNARE).Syntaxins: a family of membrane proteins acting as t-SNAREs.
Some syntaxins bind to a member of the SNAP-25 familyto form a heterodimeric t-SNARE.
VAMP ('vesicle associated membrane protein')/synaptobrevin:a family of membrane proteins acting as v-SNAREs, discovered independently by two groups.
r e v i e w s
trends in CELL BIOLOGY (Vol. 7) October 1997

r e v i e w s
(a) MDCK cells Syn 3 Syn 2
Syn ■
(b) Pancreatic acinar cells
Syn 2
Syn 2Syn 3?
Syn 4
(c) Gastric parietal ceils
U
S t i m u l a t i o n
F I G U R E 2
Differential localization of t-SNAREs in epithelial cells. According to the SNARE hypothesis,
every membrane compartment that utilizes this fusion machinery should contain aspecific target-SNARE (t-SNARE), which allows docking and fusion only of transport
vesicles possessing a matching v-SNARE. Plasma membrane t-SNAREs appear to consist oftwo subunits: one is a member of the syntaxin family and the other a member of the
SNAP-25 family. Recently, the distribution of some syntaxin isoforms has been studied inMDCK", pancreatic acinar" and gastric parietal cells'̂ which revealed their differential
distribution at the apical and basolateral plasma membrane domains. Syntaxin 4 isrestricted to the basolateral domain in both MDCK (a) and acinar cells (b). Syntaxin 2 was
found at both domains in MDCK cells but appeared to be only apical in acinar cells.Syntaxin 3 was studied in all three cell types and was found at the apical domain in
MDCK (with some additional lysosomal localization) and possibly also in acinar cells.Interestingly, syntaxin 3 could also be detected on the large secretory granules that
ultimately fuse with the (small) apical plasma membrane of acinar cells. Moreover, inparietal cells (c), at least some syntaxin 3 was localized to the HVK*-ATPase-containingtubovesicles that fuse with the apical membrane after gastric stimulation. It is not clear
whether this intracellular pool of syntaxin 3 arises from insufficient retention duringmembrane retrieval from the apical surface or whether it has a specific function.
basolateral surfaces, respectively'"'. These disparate localizations tantalizingly suggest that the .syntaxinsserve different polarized targeting pathways andperhaps contribute to the specificity of polarized targeting. Moreover, the presence of t-SNAREs at theapical plasma membrane domain implies that thisdomain does utilize the SNARE machinery and makesit unlikely that fusion to it depends entirely on anu n r e l a t e d m e c h a n i s m .
The involvement of t-SNAREs in TGN-to-apicaldelivery was not tested by Ikonen et al. and it is possible that syntaxins 2 and/or 3 are involved in TGN-to-apical transport and transcytosis. Transport from theTGN to the apical surface might involve not NSF andthe v-SNARE VAMP/synaptobrevin themselves but
Apical possibly homologues of these proteins (homologuesof NSF have been reported recently; see Ref. 64). Itis even likely that apical fusion does not involvethe tetanus-toxin-sensitive VAMP/synaptobrevin isoforms I and II, which do not bind to .syntaxins 2
Basolateral ancl 3 (Ref. 65). An involvement of SNAREs in apicalfusion is compatible with all reported data. Therefore, we suggest that SNAREs are involved in all vesi c l e - f u s i o n e v e n t s w i t h b o t h t h e b a s o l a t e r a l a n d t h e
apical plasma membrane and that the specificity offusion depends on the utilization of different isof o r m s o f t h e c o n s t i t u e n t s o f t h e S N A R E m a c h i n e r i e s .
C o n c l u s i o n s
Recent studies have uncovered several possiblemechanisms that may provide cells with the toolsnecessary for the polarized targeting of membraneproteins and lipids. Association with lipid raftswould be an elegant sorting mechanism, but thephysiological significance of 'rafting' still ret]uiresfurther experimental confirmation. It is becomingincreasingly clear that the SNARE machinery, besides being a membrane-fusion machinery, may playa role in ensuring the specificity of vesicle fusion asa final proofreading mechanism. Careful regulationof these mechanisms - e.g. by changes in lipidmetabo l ism, red is t r ibu t ion o f v - and/or t -SNAREs,utilizing different motors and/or different coat proteins - may yield the plasticity needed to generatethe 160 different major human cell types. Considerable challenges for the future will be to determinewhether our current hypotheses are true and, if so,how each o f these mechan isms func t ions in molecular terms and how they are regulated.
R e f e r e n c e s
1 MOSTOV, K. E. et al. (1995) Cold Spring Harbor Symp. Quant.Biol. 60, 775-781
2 HAMMERTON, R. W., KRZEMINSKI, K. A., MAYS, R. W.,RYAN, T. A., WOLLNER, D. A. and NELSON, W. j. (1991)Science 254, 847-850
3 MAHER, K. and MELLMAN, I. (1994) Curr. Opin.Cell Biol. 6,5 4 5 - 5 5 4
4 AROETI, B., KOSEN, P. A., KUNTZ, I. D., COHEN, F. E. andMOSTOV, K. E. (1993) /. Cell Biol. 123, 1149-1160
5 SIMONS, K. and IKONEN, E. (1997) Nature 387, 569-5726 SCHROEDER, R., LONDON, E. and BROWN, D. (1994) Proc.
Natl. Acad. Sci. U. 5. A. 91, 121 30-121347 AHMED, S. N., BROWN, D. A. and LONDON, E. Biochemistry
(in press)8 KURZCHALIA, T. V., HARTMANN, E. and DUPREE, P. (1995)
Trends Cell Biol. 5, 187-1919 MAYOR, S. and MAXFIELD, F. R. (1995) Mo/. Biol. Cell 6,
9 2 9 - 9 4 4
10 BROWN, D. A. and ROSE, |. K. (1992) Ceil 68, 533-54411 van MEER, G. (1993) Curr. Opin. Cell Biol. 5, 661-67312 van der BI]L, P., LOPES-CARDOZO, M. and van MEER, G.
(1996) /. Ce//8/0/. 132, 81 3-82113 FIEDLER, K., KOBAYASHI, T., KURZCHALIA, T. V. and
SIMONS, K. (1993) Biochemistry 32, 6365-637314 SKIBBENS, ]. E., ROTH, M. G. and MATLIN, K. S. (1989) j. Cell
Biol. 108, 821-832
15 KUNDU, A., AVALOS, R. T., SANDERSON, C. M. andNAYAK, D. P. (1996) /. Virol. 70, 6508-6515
3 9 8 trends in CELL BIOLOGY (Vol. 7) October 1997

rev iews
16 ARREAZA, C. and BROWN, D. A. (1995) /. Biol. Chem. 270,2 3 6 4 1 - 2 3 6 4 7
17 GRIFFITHS, G. and SIMONS, K. (1986) Science 234, 438-44318 KITAGAWA, Y. et al. (1994) Exp. Cell Ites. 213, 449^5719 URBAN, 1., PARCZYK, K., LEUTZ, A., KAYNE, M. and
KONDOR-KOCH, C, (1987) /. Cell Biol. 105, 2735-274320 5CHEIFFELE, P., PERANEN, |. and SIM0N5, K. (1995) Nature
378, 96-9821 FIEDLER, K. and SIMONS, K. (1995) Cell 81, 309-31222 GRAICHEN, R., L05CH, A., APPEL, D. and KOCH-BRANDT, C.
(1996) /. Biol. Chem. 271, 15854-1585723 MAYS, R. W., SIEMERS, K. A., FRITZ, B. A., LOWE, A. W.,
van MEER, G. and NELSON, W.). (1995) j. Cell Biol. 130,1 1 0 5 - 1 1 1 5
24 ZURZOLO, C., van't HOF, W., van MEER, G. and
RODRIGUEZ-BOULAN, E. (1994) EMBO /. 13, 42-5325 ZURZOLO, C,, LISANTI, M, P, GARAS, I. W., NITSGH, L. and
RODRIGUEZ-BOULAN, E. (1993) /. Cell Biol. 121, 1031-103926 NICOLAS, F, TIVERON, M. C., DAVOUST, |. and REGGIO, H,
(1994) /. Ceil Sci. 107, 2679-268927 van HELVOORT, A. et al. (1996) Cell 87, 507-51728 HARDER, T. and SIMONS, K. (1997) Curr. Opin. Cell Biol. 9,
5 3 4 - 5 4 2
29 HARDER, T., KELLNER, R,, PARTON, R. G. and GRUENBERG, J.
(1997)Mo/. 8/0/. Cetf 8, 533-54530 ZURZOLO, C., le BIVIC, A., QUARONI, A., NITSGH, L. and
RODRIGUEZ-BOULAN, E. (1992) EMBO /.II, 2337-234431 MATTER, K., BRAUCHBAR, M., BUGHER, K. and HAURI, H-P.
(1990) Cell 60, 429^3732 le BIVIG, A., QUARONI, A., NICHOLS, B. and
RODRIGUEZ-BOULAN, E. (1990) j. Ce//S/o/. 111, 1351-136133 BARTLES,). R. and HUBBARD, A. L. (1988) Trends Biochem. Sci.
1 3 , 1 8 1 - 1 8 434 ZAAL, K. J., KOK, J. W., SORMUNEN, R., ESKELINEN, 5. and
HOEKSTRA, D. (1994) Eur. /. Cell Biol. 63, 10-1935 van GENDEREN, 1. and van MEER, G. (1995) /. Cell Biol. 131,
6 4 5 - 6 5 4
36 van IJZENDOORN, S. C., ZEGERS, M. M., KOK, |. W. andHOEKSTRA, D. (1997) /. Cell Biol. 1 37, 347-357
37 MUSCH, A., XU, H., SHIELDS, D. and RODRIGUEZ-BOULAN, E.
(1996) /. Cell Biol. 133, 543-55838 YOSHIMORI, T., KELLER, P., ROTH, M. G. and SIMONS, K.
(1996)/. Ce//8/0/. 133, 247-25639 M05T0V, K. E. and WERB, Z. (1997) Science 276, 219-22040 HARSAY, E. and BRETSCHER, A. (1995) /. Cell Biol. 131, 297-31041 LEITINGER, B., HILLE-REHFELD, A. and 5PIE5S, M. (1995)
Proc. Natl. Acad. Sci. U. S. A. 92,10109-1011342 FUTTER, C. E., CONNOLLY, C. N., CUTLER, D. F. and
HOPKINS, C. R. (1995) /. Biol. Chem. 270, 10999-1100343 HEILKER, R., MANNING-KRIEG, U., ZUBER,). F. and
5PIESS, M. (1996) EMBO /. 15, 2893-289944 ROBINSON, M. S. (1997) Trends Cell Biol. 7, 99-10245 SIMPSON, F., PEDEN, A. A., CHRISTOPOULOU, L. and
ROBINSON, M. S. (1997) /. Cell Biol. 137, 835-84546 DELL'ANGELICA, E. C., OHNO, H., 001, C. E.,
RABINOVICH, E., ROCHE, K. W. and BONIFACINO,). S. (1997)EMBO I. 16, 917-928
47 MUSCH, A., COHEN, D. and RODRIGUEZ-BOULAN, E. (1997)/. Cell Biol. 138, 291-306
48 IKONEN, E., PARTON, R. G., LAFONT, F. and SIMONS, K.
(1996) Mol. Biol. Cell?, 961-97449 BECK, K. A. and NELSON, W. |. (1996) Am. j. Physiol. 270,
C 1 2 6 3 - C 1 2 7 0
50 COLE, N. B. and LIPPINCOH-SCHWARTZ, |. (1995) Curr. Opin.Cell Biol. 7, 55-64
51 LAFONT, F., BURKHARDT, |. K. and 5IM0NS, K. (1994) Nature372, 801-803
52 FATH, K. R., TRIMBUR, G. M. and BURGESS, D. R. (1994) /. CellBiol. 126, 661-675
53 MAPLES, C.)., RUIZ, W. G. and APODACA, G. (1997) /. Biol.Chem. 272, 6741-6751
54 LADINSKY, M. S., KREMER, |. R., FURCINITTI, P. S.,
MclNTOSH, J. R. and HOWELL, K. E. (1994)/. Cell Biol. 127,2 9 - 3 8
ROTHMAN, |. E. and WARREN, G. (1994) Curr. Biol. 4, 220-233
IKONEN, E., TAGAYA, M., ULLRICH, 0., MONTECUCCO, C.and SIMONS, K. (1995) Ce//81, 571-580
57 FIEDLER, K., LAFONT, F., PARTON, R. G. and SIMONS, K.
(1995)/. Cell Biol. 128, 1043-105358 APODACA, G., CARDONE, M. H., WHITEHEART, S. W.,
DASGUPTA, B. R. and MOSTOV, K. E. (1996) EMBO /. 15,1 4 7 1 - 1 4 8 1
59 RAVICHANDRAN, V., CHAWLA, A. and ROCHE, P. A. (1996)/. Biol. Chem. 271,13300-1 3303
60 WEIMBS, T., LOW, 5. H., CHAPIN, 5. ]., MOSTOV, K. E.,BUCHER, P. and HOFMANN, K. (1997) Proc. Natl. Acad. Sci.U. S. A 94, 3046-3051
LOW, S. H., CHAPIN, S.)., WEIMBS, T., KOMUVES, L. G.,
BENNETT, M. K. and MOSTOV, K. E. (1996) Mol. Biol. Cell?,2 0 0 7 - 2 0 1 8
GAISANO, H. Y. et al. (1996) Mol. Biol. Cell 7, 2019-2027PENG, X-R., YAO, X., CHOW, D-C., FORTE, ]. G. andBENNETT, M. K. (1997) Mol. Biol. CellS, 399^07
MELLMAN, 1. (1995) Ce//82, 869-87265 CALAKOS, N., BENNEH, M. K., PETERSON, K. E. and
SCHELLER, R. H. (1994) Science 263, 1146-1149
5 5
5 6
61
6 2
6 3
6 4
AcknowledgementsW e t h a n k
Kal Simons,Dick Hoekstra,Alan Marmorstein,D e b b i e B r o w n a n d
several anonymousreviewers forcomments on the
manuscript. Workin the authors'laboratory issupported bypostdoctoralfellowships fromthe IrvingtonI n s t i t u t e f o r
Immunology toT. W. and S. H. L.,
by a Feodor LynenFellowship of theA l e x a n d e r v o n
H u m b o l d t
F o u n d a t i o n t o
T. W., by anA m e r i c a n C a n c e r
Societypostdoctoralfellowship (PF3666)and an NIHi n s t i t u t i o n a l N R S A
(T32HL07731)toS. 1. C. and byNIH grantsR01 AI25144,AI39161, HL55980and AI36953 toK. E. M. We
apologize to thosea u t h o r s w h o s e
w o r k w e c o u l d n o t
cite directly owingto spacel i m i t a t i o n s .
Special Issue next monthIn November, we will concentrate on developmental cell biology in a special Issue highlightingvarious aspects of developmental biology that arenow being studied at a cell-biological level. Thisis a large field, and the articles are intended toillustrate a selection of areas In which there hasbeen recent progress rather than provide a comprehensive overview.In addition to the review articles, there will be afeature on the use of oligos to knock out maternal transcripts, a report on the joint American andInternational Society for Developmental Biologymeeting and lots of good pictures!
The reviews will include:Growth factors: a role in guiding axons?
by Sarah McFarlane and Christine HoltKnowing in your heart what's rightby Deepak Srivastava and Eric OlsonThe Notch receptor and its ligandsby Robert Fleming, Karen Purcell and
Spyros Artavanis-TsakonasControl of EGF receptor activation
in Drosophilaby jonathan Wasserman and Matthew Freeman
Expanding insights into cell proliferationin plant development
by Steven Clark and john Schiefelbein
trends in CELL BIOLOGY (Vol. 7) October 1997 3 9 9