“Next Generation Immunohistochemistry” · 2019. 4. 26. · Comparison of NG-IHC (VE1) v....
Transcript of “Next Generation Immunohistochemistry” · 2019. 4. 26. · Comparison of NG-IHC (VE1) v....
-
“Next Generation Immunohistochemistry”
A Window Onto The Molecular Biology of GI Tract Tumors
Allen M. Gown, M.D. Medical Director and Chief Pathologist
PhenoPath Laboratories Seattle, Washington
Clinical Professor of Pathology, University of British Columbia
-
Hematoxylin & EosinH & E
-
Immuno histochemistry
-
Immunohistochemistry
The application of antibodies with predefined specificities to tissue coupled with the use of detection systems permitting
visualization of the target
-
Albert Coons
American pathologist and immunologist
1912-78
-
J Clin Pathol 27:14-20, 1974
-
Taylor CR and Kledzik G Hum Pathol 12:590-6, 1981
The application of ‘immunostains’ provides an independent method of cell identification against which traditional subjective morphologic criteria may be compared: histopathology may thereby be transformed from something of an art to more of a science.
-
Cell Type Analysis Has Driven IHC Development • Immunohistochemistry can identify cell type
with greater certainty than H&E-based morphologic patterns
• Most of tumor classification based upon cell type (e.g., squamous cell carcinoma, neuroendocrine carcinoma, acinar cell tumor, etc.)
• Cell (tumor) type is a surrogate for predicting the behavior of tumor
-
Immunohistochemistry
-
Cell Type Analysis Has Driven IHC Development
Marker Normal Tissues Tumor
CDX-2 Colorectal epithelium Colorectal adenocarcinoma
SALL4 Germ cells Germ cell tumor
kit Interstitial cells Cajal GISTs
CD20 B cells B cell lymphoma
Villin GI tract epithelium GI tract adenoCAs
Insulin Beta cells of pancreatic islet Insulinoma
Synaptophysin Neuroendocrine cells of intestine Carcinoid tumor
-
And now for something completely different….
-
Next Generation Immunohistochemistry
PROVIDING A WINDOW ONTO THE MOLECULAR
ALTERATIONS UNDERLYING CANCERS AND THUS
IDENTIFYING APPROPRIATE THERAPIES
-
SCIENCE VOL 339 29 MARCH 2013
-
Major Genetic Alterations in Cancer
Mutation
Translocation
Deletion
Amplification
Methylation
-
Major Genetic Alterations in Cancer
Mutation
Translocation
Deletion
Amplification
Methylation
Loss of Expression
Abnormal Localization
Overexpression
Expression of Fusion Proteins
Mutant Protein
-
Major Genetic Alterations in Cancer
Mutation
Translocation
Deletion
Amplification
Methylation
Loss of Expression
Abnormal Localization
Overexpression
Expression of Fusion Proteins
Mutant ProteinMutation Mutant Protein
Loss of Expression
Abnormal Localization
Overexpression
-
Examples of Gene Mutations Identifiable by Immunohistochemistry
• Mutant protein (e.g.,BRAF) • Loss of expresssion (e.g, MMR, SDH) • Abnormal localization (e.g., ß-catenin) • Overexpression (e.g., p53)
-
RAS/RAF/MEK/ERK Pathway
-
BRAF• Second RAF paralogue • Proto-oncogene encoding a serine/threonine kinase
that transduces regulatory signals through the RAS/MEK/ERK pathology
• This pathway hyperactivated in ~30% of human malignancies
• Gain of function mutations result in aberrant activation of ERK signaling (thyroid papillary carcinoma, melanoma, colon carcinoma, others)
• Mutant BRAF acts as an oncogene, promoting tumor cell viability and cell growth
-
BRAF Mutations
• Activating mutations mostly in CR3 domain, in P-loop and activating segment of the kinase domain
• Most common activating mutation is thymine—>adenine in nucleotide position 1799, resulting in substitution of valine by glutamate
-
BRAF and Colorectal Cancer
• BRAF mutations (predominantly V600E) occur in 8-10% of colorectal adenocarcinomas
• BRAF and KRAS mutations are mutually exclusive
• Patients with BRAF mutated tumors have significantly shorter median progression-free and median overall survival than patents with wild type BRAF tumors
-
BRAF and Colorectal Cancer
Targeted therapies in the RAS/MEK/ERK pathology
-
BRAF V600E Mutation
• Most common BRAF mutation • Melanoma (40-60%) • Papillary thyroid carcinoma (45%) • Low grade serous ovarian carcinoma
(35%)
• Colorectal adenocarcinoma (5-15%)
-
Significance of BRAF Mutation in Colorectal Cancer
• Associated with proximal location, higher age, female, MSI-H, high tumor grade, mutinous histology
• Associated with reduced overall and disease-free survival; role in MSI-H setting uncertain
• Poor prognosis in all groups of advanced colorectal cancer
• Meta-analysis of 26 studies show mortality HR = 2.25
-
Significance of BRAF Mutation in Colorectal Cancer
• BRAF V600E mutation occurs in two thirds of MSI sporadic tumors, almost never in setting of Lynch Syndrome
• BRAF V500E in non-MSI tumors has particularly poor prognosis, mutually exclusive with KRAS mutation, but appears to predict worse or no response to EGFR targeted therapies
-
Significance of BRAF Mutation in Colorectal Cancer
• American National Comprehensive Cancer Network now recommending BRAF testing in setting of wild type KRAS metastatic CRC
-
Genes, Chromosomes & Cancer 52:748-52, 2013
• N = 31 colon cancers (14 BRAF V600E +, 17 BRAF V600 E - by pyrosequencing)
• Clone VE1 employed on Ventana platform
-
• 100% sensitivity (14/14 BRAF V600E positive tumors IHC positive)
• 100% specificity (17/17 BRAF V600E-negative tumors IHC negative)
• In minority of cases, staining intensity for mutated tumor samples weak or heterogeneous; 8/14 cases diffuse and strong
Genes, Chromosomes & Cancer 52:748-52, 2013
-
Cancer 119:2765-70, 2013
VE1
pBR1
BRAF V600EBRAF-WT BRAF V600E
-
Sinicrope FA et al., Cancer 119:2765-70, 2013
VE1
pBR1
BRAF V600E BRAF-WT BRAF-WT
-
• BRAF IHC may outperform PCR MassArray in routine clinical setting
• IHC provided “correct” results in 200/201 (99.5%) of cases, whereas MassArray was “correct” in 195/201 (97%)
Am J Surg Pathol 37:1592-1602, 2013
-
MSS and V600E BRAF
MSS and WT BRAF
Toon CW et al., Am J Surg Pathol 37:1592-1602, 2013
BRAF V600E MLH1
-
MSI and V600E BRAF
MSI and WT BRAF
Toon CW et al., Am J Surg Pathol 37:1592-1602, 2013
BRAF V600E MLH1
-
• Presence of BRAF V600E mutation in MSI colorectal carcinomas virtually excludes Lynch syndrome
• Presence of BRAF V600E mutation in MSS colorectal carcinomas predicts poor prognosis
Toon CW et al., Am J Surg Pathol 37:1592-1602, 2013
-
Proposed Algorithm
Toon CW et al., Am J Surg Pathol 37:1592-1602, 2013
MMR IHC BRAF IHC
-
Histopathology 63:187–193, 2013
• N = 52 colon cancers (17 BRAF V600E+) • Only 12/17 (71%) of BRAF V600E+ tumors
were IHC positive
• Weak cytoplasmic signal (1+) seein in 6/17 (17%) of BRAF wild type tumors
-
Adakapara CA et al., Histopathology 63:187–193, 2013
INCOMPLETE SENSITIVITY
INCOMPLETE SPECIFICITY
-
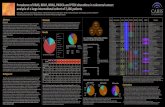
Comparison of NG-IHC (VE1) v. Molecular Analysis for Detection of BRAF V600E
Paper N No. Mutant
HIER Platform Scoring Sensitivity Specificity
Adackapara et al., 2013 52 17
Citrate pH 6
Manual S, M, W 71% 74%
Affolter et al, 2013 31 14
EDTA pH 9
Ventana Binary 100% 100%
Capper et al., 2013 91 11 pH 8 Ventana
Binary (>80%)
100% 91%
Kuan et al., 2014 128 57 pH 8 Ventana S, M, W 100% 94%
Sinicrope et al., 2013 75 25 ? Ventana S, M, W 100% 100%
Toon et al 2013 201 38 ? ?
Binary (>75%)
98% 100%
-
Comparison of NG-IHC (VE1) v. Molecular Analysis for Detection of BRAF V600E
Paper N No. Mutant
HIER Platform Scoring Sensitivity Specificity
Adackapara et al., 2013 52 17
Citrate pH 6
Manual S, M, W 71% 74%
Affolter et al, 2013 31 14
EDTA pH 9
Ventana Binary 100% 100%
Capper et al., 2013 91 11 pH 8 Ventana
Binary (>80%)
100% 91%
Kuan et al., 2014 128 57 pH 8 Ventana S, M, W 100% 94%
Sinicrope et al., 2013 75 25 ? Ventana S, M, W 100% 100%
Toon et al 2013 201 38 ? ?
Binary (>75%)
98% 100%
Why This Discordance?
-
Hum Pathol 45: 464-72, 2014
• Prospective study of 103 cases (57 with BRAF V500E mutation)
• 100% sensitivity, 94% specificity • Suggest that reason for discordance in other
studies may be different epitope retrieval methods
-
Potential Reasons
•Different tissues and fixation •Different epitope retrieval methods •Different detection systems and
platforms
•Different observers • First corollary to Gown’s 2nd Law of IHC
-
Always Employ Antibodies Within The First Six Months of Their Publications That’s When They Are Most Specific!
-
BRAF V600E
• Immunohistochemistry can be employed to see the mutant BRAF protein in the cytoplasm of tumor cells
• Immunohistochemistry may or may not be adequately sensitive and specific to replace, or be integrated with, molecular assays
s u m m a r y
-
Examples of Gene Mutations Identifiable by Immunohistochemistry
• Mutant protein (e.g.,BRAF) • Loss of expresssion (e.g, MMR, SDH) • Abnormal localization (e.g., ß-catenin) • Overexpression (e.g., p53)
-
Examples of Mutations Leading to Loss of Protein Expression
INI-1/SMARCB1Rhabdoid tumors
(and others)
Mismatch Repair (MLH1, MSH2, MSH6, PMS2)
Coloretal adenocarcinoma
E-cadherinLobular breast
cancer
Succinic dehydrogenase
Subset of gastrointestinal stromal tumors
PTENEndometrial, breast cancer
-
Reasons for MMR IHC
Identifying Lynch Syndrome patients
Identifying patients with sporadic ‘MSI tumors’ (who may not require FU-based chemotherapy)
Identifying ‘carcinomas of unknown primary’ that are ‘minimally differentiated’ colorectal adenocarcinoma
-
Autosomal dominant
Mutation in MLH1 (~60%), MSH2 (~30%), or MSH6 (~10%)
Accounts for 2-5% of colorectal adenocarcinoma
Tumors develop at early age, usually found on right side
Also develop endometrial adenocarcinoma
Synchronous and metachronous colorectal cancers: 40% develop within 10 years without total colonic resection
HNPCC (Lynch Syndrome) Hereditary Non-polyposis Colorectal Cancer
-
Reasons for MMR IHC
Identifying Lynch Syndrome patients
Identifying patients with sporadic ‘MSI tumors’ (who may not require FU-based chemotherapy)
Identifying ‘carcinomas of unknown primary’ that are ‘minimally differentiated’ colorectal adenocarcinoma
-
Classical ‘Vogelstein’ Pathway of Colonic
Adenocarcinoma Progression
Figure 11.10 The Biology of Cancer (© Garland Science 2007)
-
DNA Mismatch Repair System
MLH1
PMS2
MLH2
MSH6
-
DNA Mismatch Repair
DNA mismatch repair promotes genomic stability by correcting base-base and small insertion/deletion mispairs that arise during DNA
replication and recombination
http://www.helsinki.fi/bioscience/mmrandcancer/mmrgenetics.html
http://www.helsinki.fi/bioscience/mmrandcancer/mmrgenetics.html
-
Repetitive segments of DNA two to five nucleotides in length scattered throughout the genome both in noncoding as well as coding regions
Regions are inherently unstable and susceptible to mutations
What Are Microsatellites?
-
The presence of a discrepancy between the size of microsatellites in DNA from tumor compared with nontumor tissue
Usually results from loss of gene expression of one or more MMR genes that would normally correct these errors
What Is Microsatellite Instability (MSI)?
-
MLH1
-
MSH2
-
MSH6
-
PMS2
-
New Nomenclature
dMMR pMMR
-
MSI-H tumors more likely arise on the right side
MSI-H tumors more likely to occur in people with positive family history of colorectal cancer
MSI-H tumors more likely to be cribriform, solid, signet ring, high grade (’medullary’), mucinous
Lymphocytic infiltration most important feature for predicting MSI-H (nodular “Crohn-like” peritumoral or TIL)
Are MSI-H tumors distinct?
-
Histologic Patterns of MSI Adenocarcinomas
from Bellizzi AM and Frankel WL, Adv Anat Pathol 16:405-17, 2009
Mucinous Signet Ring
Medullary
MucinousMedullary
TIL Pushing Border
-
IHC vs. MSI Testing
Cost $$ $$$
Analyte Protein DNA
How much tumor required Very little Very little
Requirements Tumor only Tumor + normal
Possibility of contamination by normal No Yes
Turnaround Next day 2-7 days
Identifies involved gene Yes No
Assay sensitive to fixation Yes No
adapted from Bellizzi AM and Frankel WL, Adv Anat Pathol 16:405-17, 2009
IHC MSI
-
IHC v. MSI Testing
Concordance high in most studies
High concordance possible even with just two antibodies (e.g., MLH1, MSH2) but even higher with four (MLH1, MSH2, PMS2, MSH6)
Potential shortcoming if IHC is inability to detect missense mutations that nevertheless result in immunoreactive but nonfunctional protein
-
Rigau V et al. Arch Pathol Lab Med 127:694-700, 2003
-
MSI-Type Colorectal Adenocarcinomas
Hypermethylation of MLH1 promoter CpG islands But such tumors may be better characterized as “CpG Island Methylator Phenotype High” (CIMP-H)
-
Immunohistochemical localization “integrates” what happens at the genomic level to MMR genes
Identifies genotypically distinct variants of colorectal adenocarcinoma with important clinical implications
MMR IHC and Colorectal Adenocarcinoma
-
Ribic CM et al. NEJM 349:247-57, 2003
NO ADJUVANT CHEMOTHERAPY
N=570
-
Ribic CM et al. NEJM 349:247-57, 2003
ADJUVANT CHEMOTHERAPY
N=570
-
Outcome of Patients with Stage III Colorectal Adenocarcinoma Treated with Adjuvant 5-FU
J Clin Oncol 28:3219-26, 2010
dMMR pMMR
n = 457
-
Conflicting Data on Predictive Role of MSI in 5-FU Response in Colorectal Adenocarcinoma
Vilar E and Tabernero J, Cancer Discovery May 2013 502-11
-
Reasons for MMR IHC
Identifying Lynch Syndrome patients
Identifying patients with sporadic ‘MSI tumors’ (who may not require FU-based chemotherapy)
Identifying ‘carcinomas of unknown primary’ that are ‘minimally differentiated’ colorectal adenocarcinoma
-
Keratins [OSCAR]
-
Keratin
Keratin 7
-
Keratin
Keratin 20
-
CDX-2
CDX-2
-
CDX-2
CDX-2
-
Villin
Villin
-
Immunophenotype
Keratins [OSCAR] Uniformly positive
Keratin 7 Negative
Keratin 20 Negative
CDX2 Negative
Villin ?Focally positive
Synaptophysin Negative
-
MSH-2
-
MSH-6
-
MLH-1
-
PMS-2
-
Am J Pathol 159:2239-2248, 2001
“Minimally differentiated” or “medullary” carcinoma
87% show reduced or absent CDX2
60% showed MSI phenotype
-
Am J Clin Pathol 140:561-6, 2013
CDX2-/K20- phenotype associated with older age, higher stage, LN metastases, “medullary” histology, BRAF mutation, CIMP-H status.
Patients have worse survival compared with those expressing CDX2 and/or K20
This is a poor prognosis subgroup
-
There must be complete loss of MMR expression in the tumor cell population
There can be variegated and incomplete immunostaining owing to fixation issues as well as intrinsic variation (e.g., MSH6)
Don’t overcall dMMR if there is no staining within the non-neoplastic elements
MMR IHC Interpretation Caveats
-
Examples of Gene Mutations Identifiable by Immunohistochemistry
• Mutant protein (e.g.,BRAF) • Loss of expresssion (e.g, MMR, SDH) • Abnormal localization (e.g., ß-catenin) • Overexpression (e.g., p53)
-
Gastrointestinal Stromal Tumors (GISTs)
• Originally thought to be smooth muscle tumors (“leiomyoblastoma”) or autonomic nerve tumor (“GANT”)
• Related to interstitial cells of Cajal (ICC) • Both GISTs and ICCs express KIT,
CD34, and DOG1
-
KIT and PDGFRA Mutations in GISTs
from Marrari A et al, Arch Pathol Lab Med 136:483-9, 2012
-
c-kit IHC is a Cell Type Specific Marker!
• Is marker both of normal interstitial cells of Cajal as well counterpart tumor, gastrointestinal stromal tumor
• Presence of c-kit expression in GIST is not evidence of presence of activating mutation and hence eligibility for imatinib
-
SDH Mutations and GISTs
• Carney-Stratakis syndrome caused by germ line mutations in SDH subunits B, C, or D
• Predisposes to GISTs and paragangliomas • Investigated sporadic GISTs in patients lacking
kit or PDGFRA mutations (N =34)
PNAS 108:314-8, 2011
-
SDH-Deficient GISTs
adapted from Doyle LA and Hornick JL, Histopathol 64:53-67, 2014
Feature SDH Deficient SDH ProficientAge Children and young
adultsOlder adults
Sex distribution F > M F = MLocation Stomach Entire GI tractMultinodular Almost always RareMultifocality Common RareHistology Epithelioid or mixed Spindle commonLymph node mets Common RareCourse of mets Indolent AggressiveImatinib sensitivity No Most casesc-kit positive IHC Yes Yesc-kit mutations None ~95%SDH mutations ~50% None
-
SDH Mutations and GISTs
Janeway KA et al., PNAS 108:314-8, 2011
-
SDH Mutation GISTs
Multinodular architecture
Mixed spindle and epithelioid morphology
Loss of Expression of SDH-B
Doyle LA, Histopathol 61:801-9, 2012
-
GIST: Phenotype-Genotype Correlations
kit exon 11 all locations; usually spindle or mixed Excellentkit exon 9 small and large bowel; spindle or mixed Better at higher dose
kit exon 13 usually small bowel; spindle Somekit exon 17 usually small bowel; spindle Somekit exon 8 small bowel; mixed ?
PDGFRA exon 19 stomach and omentum; epithelioid PoorPDGFRA exon 12 stomach; epithelioid VariablePDFRA exon 14 stomach; epithelioid VariableSDH deficient* stomach; epithelioid or mixed PoorSDHA-mutant stomach; epithelioid or mixed Poor
SDHB/D/D mutant stomach; epithelioid or mixed Poor
Genotype Sites and Histology Imatinib response
Doyle LA and Hornick JL, Histopathol 64:53-67, 2014 *including Carney Stratakis and Carney triad
-
Succinate dehydrogenase is an enzyme complex, bound to the inner mitochondrial membrane of mammalian mitochondria and many bacterial cells. It is the only enzyme that participates in both the citric acid cycle and the electron transport chain.
http://en.wikipedia.org/wiki/Enzymehttp://en.wikipedia.org/wiki/Inner_mitochondrial_membranehttp://en.wikipedia.org/wiki/Inner_mitochondrial_membranehttp://en.wikipedia.org/wiki/Mammalianhttp://en.wikipedia.org/wiki/Mitochondriahttp://en.wikipedia.org/wiki/Bacterialhttp://en.wikipedia.org/wiki/Cell_(biology)http://en.wikipedia.org/wiki/Citric_acid_cyclehttp://en.wikipedia.org/wiki/Citric_acid_cyclehttp://en.wikipedia.org/wiki/Electron_transport_chain
-
Courtesy of Jason L. Hornick, MD PhD
-
KIT
Courtesy of Jason L. Hornick, MD PhD
-
SDHB
Courtesy of Jason L. Hornick, MD PhD
-
KIT
Courtesy of Jason L. Hornick, MD PhD
-
SDHBCourtesy of Jason L. Hornick, MD PhD
-
Courtesy of Jason L. Hornick, MD PhD
-
Courtesy of Jason L. Hornick, MD PhD
-
KIT exon 11-mutant GIST
SDHBCourtesy of Jason L. Hornick, MD PhD
-
Examples of Gene Mutations Identifiable by Immunohistochemistry
• Mutant protein (e.g.,BRAF) • Loss of expresssion (e.g, MMR, SDH) • Abnormal localization (e.g., ß-catenin) • Overexpression (e.g., p53)
-
49 year old male presents with 5 cm mesenteric mass 2 years after partial gastrectomy for
GIST
-
DIFFERENTIAL DIAGNOSES
• Recurrent gastrointestinal stromal tumor
• Other (myofibroblastic process?desmoid?)
-
CD34
-
CD117
-
SMActins [1A4]
-
ß-catenin
-
Mesenteric Fibromatosis• Aggressive fibromatosis, desmoid tumor
• All ages
• Associated with Gardner syndrome
• Abdominal and extra-abdominal (shoulder, chest wall, back)
• Deep-seated, poorly circumscribed
• Most present with asymptomatic abdominal mass
-
ß-Catenin: Role in Cell Adhesion and Signaling
Axin and APC are negative regulators of
Wnt signalling cascade. Axin and APC phosphorylate ß-catenin on APC
binding sites, thereby degrading and inactivating the
protein.
Regulation of ß-catenin critical to
APC’s tumor suppressor effect
-
Nuclear beta catenin
• In nucleus, beta catenin interacts with transcription factors of TCF/LEF family and thus takes part in alteration of gene expression
• ß-catenin in nucleus continuously drives transcription of target genes
• Lead to increased cell proliferation and/or inhibition of apoptosis
-
ß-Catenin
-
ß-Catenin and Fibromatoses
• Montgomery et al, AJSP, 2002
• Fibromatoses have mutation in APC/ ß-catenin pathway
• Abnormal nuclear accumulation of ß-catenin protein
• Studied expression by IHC in mesenteric fibromatosis, GIST, and sclerosing mesenteritis
-
Meenteric Fibromatosis
ß-Catenin
Mesenteric Fibromatosis
-
Replace with new photos
ß-Catenin
-
ß-Catenin
GIST
-
Modern Pathology 18:68-74, 2005
Is Nuclear ß-catenin Expression Found in
Other Tumors?
-
Ng TL et al., Modern Pathology 18:68-74, 2005
-
Ng TL et al., Modern Pathology 18:68-74, 2005
Tumors POSITIVE for high level nuclear ß-catenin expression
• Desmoid type fibromatosis (71%)
• Solitary fibrous tumor (40%)
• Endometrial stromal sarcoma (40%)
• Synovial sarcoma (28%)
-
Abnormal localization of ß-catenin to nucleus
• May be mutation of ß-catenin or adenosis polyposis coli (APC) genes
• APC mutations more common in setting of familial adenomatous polyposis
• ß-catenin mutations more common in sporadic aggressive fibromatosis
• Demonstrates that mutation of one gene may result in abnormal localization of another gene product
-
Nuclear beta catenin in colorectal carcinoma
• May be a consequence of inactivating mutation of APC tumor suppressor gene which is now unable to down-regulate intracellular beta catenin
• OR activation of beta catenin by somatic mutations
• Accumulation of nuclear beta catenin may be first visible consequence of loss of APC function
-
‘Chromosomal Instability’ Pathway
-
Early sign of carcinoma?
• Detection of nuclear ß-catenin in adenomas represents expansion of cells - estimate malignant potential?
• PJ polyps, which have no APC gene mutations, do not accumulate nuclear ß-catenin
-
Even earlier than adenoma?[CANCER RESEARCH 61, 8085– 8088, November 15, 2001]
-
ß-Catenin
• Immunohistochemistry can be employed to see the nuclear ß-catenin protein abnormally located in the nucleus
• Nuclear ß-catenin is a surrogate marker for the presence of APC or ß-catenin mutations
s u m m a r y
-
Examples of Gene Mutations by Identifiable by Immunohistochemistry
•Mutant protein (e.g.,BRAF) • Loss of expresssion (e.g, MMR, SDH) •Abnormal localization (e.g., ß-catenin) •Overexpression (e.g., p53)
-
Identifying Mutated p53 in Human Tumors
Bártek J, Bártková J, Vojtĕsek B, Stasková Z, Lukás J, Rejthar A, Kovarík J,Midgley CA, Gannon JV, Lane DP. Aberrant expression of the p53 oncoprotein is a common feature of a wide spectrum of human malignancies. Oncogene 6:1699-1703, 1991 p53
Applications to Esophageal AdenoCA
-
p53• Encoded by TP53 gene • “Guardian of the genome” function as tumor
suppressor
• Can activate DNA repair proteins following DNA damage (e.g., radiation)
• Can arrest cell growth by holding cells at G1/S regulation point
• Can initiate process of apoptosis (programmed cell death) if DNA damage proves to be irreparable
-
p53
-
p53• Li-Fraumeni syndrome patients have only one
functional copy of p53, develop tumors in early adulthood
• Mutations can develop following exposure to chemicals, radiation, viruses, etc.
• More than 50% of human tumors contain mutations or deletions in TP53
• Most common is missense mutation involving exons 5-8 coupled with loss of wild type allele (LOH)
-
p53 Immunohistochemistry
•Rapid • Inexpensive •Widely available • Surrogate marker for mutational p53
status?
-
p53• Most (but not all) inactivating mutations result in
conformational change of p53 molecule that results in prolonged half-life
• Half-life 20 minutes for wild type, hours for mutant proteins
• Wild type protein detectable by immunohistochemistry but at low levels that seem to correlate with cell proliferation
• Large deletions or truncating mutations may result in apparent loss of p53 expression
-
p53 and Cancer• Missense mutations would be predicted to
correlate with nuclear overexpression
• Approximately one-third of TP53 mutations are null (nonsense, frameshift, splice site mutations) probably resulting in complete absence of protein expression
• Deletions would also predict to result in complete absence of protein expression
• Might expect three immunostaining patterns
-
J Pathol 222:191-8, 2010
• DO-7 anti-p53 monoclonal antibody (cross reacts with wild type and mutant)
• Scored in three bins: complete absence of expression, focal expression, overexpression (>50%)
• Outcome in two different cohorts
-
p53 and Ovarian Cancer
Kobel M et al., J Pathol 222:191-8, 2010
-
p53 and Ovarian Cancer
• Pelvic high grade serous ovarian cancers show either complete loss or overexpression in 88% of cases
• p53 overexpression associated with reduced risk of recurrence
• Complete absence of expression associated with unfavorable outcome
• Suggests functional differences underlying overexpression v. absence of expression
Kobel M et al., J Pathol 222:191-8, 2010
-
p53 ImmunohistochemistryThree unique immunostaining patterns
Mutated
Mutated
Wild type
-
p53 and Barrett’s
• N = 154 biopsy specimens with Barrett’s, 32 specimens without dysplasia
• p53 immunohistochemistry assists in diagnosis in difficult cases and predicts progression
Kaye PV, et al. Histopathol 54:699-712, 2009
-
Suggested Algorithm
Kaye PV, et al. Histopathol 54:699-712, 2009
-
• Low grade dysplasia currently only accepted predictor for neoplastic progression in Barrett’s esophagus
• Can alterations in p53 improve risk stratification?
Gut 62:1676-83, 2013
-
Barrett’s esophagus with
low grade dysplasia
Barrett’s esophagus with
low grade dysplasia
Esophageal adenoCA with
complete loss of p53
-
Kastelein F et al., 2013
•N = 635 patients •Retrospective study of p53 protein expression
as determined by IHC
•8% of patients developed high grade dysplasia or adenocarcinoma
•More powerful predictor than histologic diagnosis of LGD
•Strongly associated with overexpressioln and especially loss of p53 expression
-
Kastelein F et al., 2013
Incidence of p53 Overexpression and Absence of Expression
-
Gut 63:7-42, 2014
• Marker with greatest body of evidence which can also be applied routinely is p53
• 50-89% positive in Barrett’s dysplasia • Can improve inter observer variability for reporting
dysplasia (especially LGD v. atypical reactive [ID])
• Powerful predictor of progression (OR 3-8)
-
Major Genetic Alterations in Cancer
Mutation
Translocation
Deletion
Amplification
Methylation
Loss of Expression
Abnormal Localization
Overexpression
Expression of Fusion Proteins
Mutant Protein
Translocation
Overexpression
Expression of Fusion Proteins
-
Examples of Chromosomal Translocations Identifiable by NG-IHC
Tumor Translocation Fusion Generated NG-IHC Target
PNET/ES t(11;22)(q24;q12) EWSR1-FLI1 FLI1
ALCL t(2;5)(p23;q35) NPM-ALK ALK
ASPS der(17)t(X;17)(p11;q25) ASPL-TFE3 TFE3
Synovial sarcoma t(X;18)(p11.2;q11.2) SYT-SSX1 TLE-1
DSRCT t(11;22)(q11;q12) EWSR1-WT1 WT-1
AML t(8;21)(q22;q22) AML1-ETO AML1-ETO
Lung cancer Chromosme 2 inversion EML4-ALK ALK
-
Major Genetic Alterations in Cancer
Mutation
Translocation
Deletion
Amplification
Methylation
Loss of Expression
Abnormal Localization
Overexpression
Expression of Fusion Proteins
Mutant Protein
Amplification Overexpression
-
Examples of Amplified Proteins Identifiable by NG-IHC
Tumor Protein amplified
Breast, gastric cancer HER2
Liposarcoma MDM-2, CDK4
Lymphoma bcl-2
Lung cancer EGFR
-
HER2 Overexpression in Gastric Cancer
Normal ~20-50,000 receptors
HER2 Overexpressed Up to ~2,000,000 receptors
Genentech 2010
-
Data Presented At SABCS, December 2012
Negative (0, 1+) Positive (3+)
Non-amplified
3903 (98.6%)
13
Amplified 57450
(97.2%)
TOTALS 3960 463
N = 9,022 breast cancer cases, 2008-2012
-
The Role of the Pathologist (Then)
-
• NG-IHC can be used to identify molecular alterations that characterize selected malignancies
• NG-IHC acts as a surrogate for molecular studies, and is less expensive and time consuming and, in some cases, can provide more information
Next Generation Immunohistochemistry
1
-
•NG-IHC can integrate different genotypic changes which result in the same phenotypic changes
•NG-IHC can thus expand and better define categories of disease
2
Next Generation Immunohistochemistry
-
•The paradigm shift to molecular based classification of tumors will continue and will accelerate
•Molecular oncodiagnostics will play an increasingly large role in tumor analysis
What does the Future of Pathology Look Like?
-
Patients
Oncologists
The New Paradigm of Pathology
BioPharma Targeted Therapies
Published Literature Pathology, Oncology
-
The Changing Role of the Pathologist
“As more drugs that target specific components of signal-transduction pathways become available and as we increase our knowledge of the complexity of these signalling networks, the burden of selecting the correct drug combinations for each individual cancer patient will ultimately shift to the pathologist, who must identify the underlying defect in each tumor.”
Shaw RJ and Cantley LC. Nature 441:424-30, 2006
-
Thank you for your attention
Photograph by Dave Morrow