Amino Acids
-
Upload
akash-marathakam -
Category
Documents
-
view
527 -
download
3
description
Transcript of Amino Acids

AMINO ACIDSAMINO ACIDS

TopicsTopics
IntroductionDefinitionClassificationPropertiesEssential Amino AcidSelected Synthesis of Amino Acid

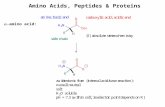
DefinitionDefinitionAmino acids are the simplest unit of a protein
molecule and they form the building blocks of protein structure.
An amino acid consists of a free NH2 and a free COOH (carboxyl) group. Both are attached to the same carbon atom. In the structure of the amino acid referred above, ‘R’ represents the groups other than the NH2 and COOH groups. This may be aliphatic or heterocyclic group. In glycine which is the simplest of the amino acids, ‘R’ represents ‘H’ atom.

In the steric configuration for serine, the COOH is written on the top, while the amino group is written to the left in the case of L-serine and to the right in the case of D-serine.

ClassificationClassification(A) Amino acids are classified in
different ways, among these, the classification referring to the number of carboxyl groups present in the amino acids and nature of structure.
◦Mono amino mono carboxylic acids◦Mono amino dicarboxylic acids◦Diamino mono carboxylic acids◦Sulphur containing amino acids







1. Essential amino acids
They are indispensable and are required for nutrition, promotion of normal growth and maintenance of nitrogen balance.
◦Methionine◦Arginine◦Threonine◦Valine◦Isoleucine◦Leucine◦Phenylalanine◦Lysine◦Histidine◦Tryptophan
II. Non Essential amino acid
B. Metabolism of Individual Amino Acids

PropertiesPropertiesPhysical Properties
1. Amino acids are white crystalline substances.
2. Generally soluble in water & insoluble in organic solvents.
3. High melting points, varies from 2000oC-3000oC or even more.
4. They may be tasteless, sweet or bitter.5. All amino acids except glycine are optically
active.6. They are amphoteric, react with both
alkalies & acids. They carry +ve charge in acids, but –ve charge in alkaline solution. But they are electrically neutral in certain reaction around neutrality.

Zwitterion Theory◦According to this theory, amino acids
possessing double charges (positive and negative) are called zwitterions.
◦Isoelectric point ◦The PH at which amino acid has no
tendency to move either to the positive or negative electrode is called its isoelectric point

Chemical PropertiesThe chemical properties of amino acids are
due to their amino groups and carboxyl groups, so they classified as follows,
1. Reaction of amino acids due to amino groups
2. Reaction of amino acids due to carboxyl groups

(1) Reaction of amino acids due to amino groupsReaction with formaldehyde
Reaction of Glycine with Benzoic acid
Reaction with Nitrous acid

Reaction of NinhydrinOxidative deamination of amino acid
The blue coloured compound Rheumann’s purple formed as follows,

Reaction with I-Fluro-2, 4-Dinitrobenzene (FDNB):
Reaction with carbon dioxide-Siegfried’s carbamino reaction:

Formation of Diketopiperazines:
Chelation with metal ions:
◦Heavy metals like Cu++, Hg++, Mn++, Fe++, etc. form chelated complexes with amino acids in which both carboxyl & amino groups are involved.

Formation of Esters:
Decarboxylation-Formation of Amine:
(2) Reaction of amino acids due to carboxyl groups

Formation of Amide:
Amino acid esters form amides when treated with anhydrous or alcoholic ammonia

Amination of α–halogenated acids.◦ An α -chloro- or bromo-acid is treated with concentrated ammonia,
◦ Gabriel’s phthalimide synthesis with α-halogeno-acids
Selected Synthesis of Amino AcidSelected Synthesis of Amino Acid

•Aromatic aldehydes with Aromatic aldehydes with diketopiperazinediketopiperazine

ReferencesReferencesFundamentals of Biochemistry for
Medical Students by Ambika Shanmugam
Organic Chemistry Volume II:Steriochemistry and the Chemistry of Natural Products by I.L.Finar

You have downloaded this presentation from akashmarathakam’s scribd pageYou have downloaded this presentation from akashmarathakam’s scribd page