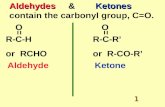
Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
Aldehydes and Ketones Chapter 14. Structure Aldehydes and ketones contain a carbonyl group which...
-
Upload
randall-thomas -
Category
Documents
-
view
233 -
download
0
Transcript of Aldehydes and Ketones Chapter 14. Structure Aldehydes and ketones contain a carbonyl group which...

Aldehydes and KetonesAldehydes and Ketones
Chapter 14Chapter 14

StructureStructure Aldehydes and ketones contain a carbonyl Aldehydes and ketones contain a carbonyl
group which consists of a carbon double-bonded group which consists of a carbon double-bonded to an oxygen.to an oxygen.
In aldehydes, the carbonyl group is at the end.In aldehydes, the carbonyl group is at the end. In ketones, the carbonyl group is located within In ketones, the carbonyl group is located within
the chain.the chain.
C
O
C
H R
O
C
R R
O
Aldehyde Ketone

PropertiesProperties
Polar compoundsPolar compounds Boiling points higher than ethers and Boiling points higher than ethers and
hydrocarbons but less than alcoholshydrocarbons but less than alcohols Alkanes < Ethers < Aldehydes and Ketones < AlcoholsAlkanes < Ethers < Aldehydes and Ketones < Alcohols
Aldehydes and ketones with less than five Aldehydes and ketones with less than five carbons are soluble in water.carbons are soluble in water.

Properties Practice Problem 1Properties Practice Problem 1
Which member in each pair would have a higher Which member in each pair would have a higher boiling point?boiling point?
A.A.
B. B.
CH3COH
O
CH3CCH3
O
CH3CH2CH2CH3 CH3CH2CH
O

Properties Practice Problem 1 Properties Practice Problem 1 AnswerAnswer
Which member in each pair would have a higher Which member in each pair would have a higher boiling point?boiling point?
A.A.
B. B.
CH3COH
O
CH3CCH3
O
CH3CH2CH2CH3 CH3CH2CH
O

Properties Practice Problem 2Properties Practice Problem 2
Which member in each of the following pairs will Which member in each of the following pairs will be more water soluble?be more water soluble?
A.A.
B. B.
CH3CH2CH2CH3 CH3CH2CH
O
CH3CH2CH2CCH2CH2CH3
O
CH3CH2CH
O

Properties Practice Problem 2Properties Practice Problem 2
Which member in each of the following pairs will Which member in each of the following pairs will be more water soluble?be more water soluble?
A.A.
B. B.
CH3CH2CH2CH3 CH3CH2CH
O
CH3CH2CH2CCH2CH2CH3
O
CH3CH2CH
O

IUPAC Nomenclature of AldehydesIUPAC Nomenclature of Aldehydes
Determine the parent chain which is the Determine the parent chain which is the longest continuous chain containing the longest continuous chain containing the carbonyl group.carbonyl group.
Replace the final –e with –al.Replace the final –e with –al. Number the chain beginning with the Number the chain beginning with the
carbonyl carbon.carbonyl carbon. Number and name all substituents as Number and name all substituents as
usual.usual.

NAMING ALDEHYDES THE NAMING ALDEHYDES THE IUPAC WAYIUPAC WAY
See page 399 question 14.3:See page 399 question 14.3:
CHCH33 O O
a)a) CHCH33CHCHCHCHCHCH22-C-H-C-H
CHCH33
OO
b)b) CHCH33CH-C-HCH-C-H
ClCl

Naming Aldehydes the IUPAC Naming Aldehydes the IUPAC wayway
OO
c)c) CHCH33CHCH22CHCH22CH-C-HCH-C-H
CHCH22CHCH33
OO
d)d) CHCH33CHCHCHCH22-C-H-C-H
OHOH

Common Names for AldehydesCommon Names for Aldehydes
Formaldehyde AKA methanalFormaldehyde AKA methanal Acetaldehyde AKA ethanalAcetaldehyde AKA ethanal
Others can be found in Table 14.1 on pg. Others can be found in Table 14.1 on pg. 398398

IUPAC Nomenclature for KetonesIUPAC Nomenclature for Ketones
Very similar to naming aldehydesVery similar to naming aldehydes The –e ending is replaced with –one.The –e ending is replaced with –one. The location of the carbonyl carbon is The location of the carbonyl carbon is
indicated with a number.indicated with a number. The longest chain is numbered to give the The longest chain is numbered to give the
carbonyl carbon the lowest possible carbonyl carbon the lowest possible number.number.

Naming Ketones the IUPAC Naming Ketones the IUPAC wayway
See page 401 question 14.5See page 401 question 14.5
OO O O
a)a) CHCH33CH-C-CHCH-C-CH33 b) CHb) CH33CH-C-CHCH-C-CH33
II CH CH33
OO OO
c) CHc) CH33CH-C-CHCH-C-CH22CHCH33 d) CHd) CH33CHCHCHCH22-C-CH-C-CH33
FF CH CH22CHCH22CHCH22CHCH3

Common Nomenclature for Common Nomenclature for KetonesKetones
Propanone is also known as acetone.Propanone is also known as acetone. Name the alkyl groups that are bonded to Name the alkyl groups that are bonded to
the carbonyl carbon.the carbonyl carbon. Use these as prefixes followed by the Use these as prefixes followed by the
word ketone.word ketone. Arrange the alkyl groups alphabetically.Arrange the alkyl groups alphabetically.

KETONES- COMMON NAMESKETONES- COMMON NAMES
OO O O
a)a) CHCH33CH-C-CHCH-C-CH33 b) CHb) CH33CH-C-CHCH-C-CH33
CHCH33
OO O O
c) CHc) CH33CH-C-CHCH-C-CH22CHCH33 d) CHCHd) CHCH22-C-CH-C-CH33
CHCH22CHCH22CHCH22CHCH

HomeworkHomework
Pg. 418: Pg. 418:
# 14.25, 26, 27, 31, 33, 35, & # 14.25, 26, 27, 31, 33, 35, & 3636