9-01 Skin Grafts
Transcript of 9-01 Skin Grafts


HISTORY
Ratner1 and Hauben, Baruchin, and Mahler2 giveexcellent overviews of the history of skin grafting.The following highlights are excerpted from thesetwo sources.
Grafting of skin originated among the tilemakercaste in India approximately 3000 years ago.1 Acommon practice then was to punish a thief oradulterer by amputating a nose, and surgeons ofthis day took free grafts from the gluteal area torepair the resulting deformities. From this modestbeginning, skin grafting evolved into one of thebasic clinical tools in plastic surgery.
In 1804 an Italian surgeon named Boronio suc-cessfully autografted a full-thickness skin graft on asheep. In 1817 Sir Astley Cooper grafted a full-thickness piece of skin from a man’s amputatedthumb onto the stump for coverage. Bunger in1823 successfully reconstructed a nose with a skingraft. On the other side of the Atlantic, JonathanMason Warren in 1840 and Joseph Pancoast in1844 grafted autogenous full-thickness skin fromthe arm to the nose and the earlobe, respectively.2
In 1869, 25 years later, Reverdin rekinkled world-wide interest in skin grafting with his report ofsuccessful pinch grafts.
Ollier in 1872 pointed out the importance ofthe dermis in skin grafts, and in 1886 Thierschused thin split-thickness skin to cover large wounds.To this day the names Ollier and Thiersch are syn-onymous with thin (0.005–0.01") split-thicknessgrafts.
Lawson, Le Fort, and Wolfe all used full-thick-ness grafts to successfully treat ectropion of thelower eyelid; nevertheless, it is Wolfe whose nameis generally associated with the concept of full-thickness skin grafting. Krause popularized theuse of full-thickness grafts in 1893, known todayas Wolfe-Krause grafts.
Brown and McDowell3 reported thick split-thick-ness grafts (0.01–0.022") for the treatment of burnsin 1942.
In 1964 Tanner, Vandeput, and Olley4 gave usthe technology to expand skin grafts with a ma-
Skin Grafts and Skin Substitutes
Philip L Kelton Jr MD
chine that would cut the graft into a lattice pattern,enlarging it up to 12X its original surface area.
In 1975 epithelial skin culture technology waspublished by Rheinwald and Green,5 and in 1979cultured human keratinocytes were grown to forman epithelial layer that was satisfactory for graftingwounds.6
ANATOMY
The character of the skin varies greatly amongindividuals and within each subject it varies withage, sun exposure, and area of the body. For thefirst decade of life the skin is quite thin, but fromage 10 to 35 or so it thickens progressively. Atsome time during the fourth decade the thicken-ing stops and the skin once again begins to de-crease in substance. From that time until the per-son dies there is gradual thinning of dermis, de-creased skin elasticity, and progressive loss of se-baceous gland content.
The skin also varies greatly from body site tobody site. Skin from the eyelid, postauricular andsupraclavicular areas, medial thigh, and upper ex-tremity is thin, whereas skin from the back, but-tocks, palms of the hands and soles of the feet ismuch thicker.
Approximately 95% of the skin is dermis, whilethe other 5% is epidermis.7 The dermis containssebaceous glands. Sweat glands and hair folliclesare located in the subcutaneous fat just beneaththe dermis. The skin vasculature is superficial tothe superficial fascia and parallels the skin surface.The cutaneous vessels branch at right angles topenetrate subcutaneous tissue and arborize in thedermis. The final destination of these blood ves-sels is a capillary tuft that terminates between der-mal papillae.
TERMINOLOGY
An autograft is a graft taken from one part of anindividual’s body that is transferred to a differentpart of the body of that same individual. An isograft

SRPS Vol 9, No 1, 1999
2
is a graft from genetically identical donor and re-cipient individuals, such as litter mates in inbredrats or identical twins in humans. An allograft (pre-viously homograft) is taken from another individualof the same species. A xenograft (heterograft) is agraft taken from an individual of one species thatis grafted onto an individual of a different species.
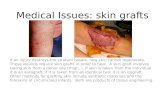
A split-thickness skin graft (STSG) contains epi-dermis and a variable amount of dermis. A full-thickness skin graft (FTSG) includes all of the der-mis as well as the epidermis (Fig 1).8 The donorsite of an FTSG must be closed either by directsuture approximation or skin graft.
PROPERTIES OF SKIN GRAFTS
Skin grafts have been used for over a century toresurface superficial defects of many kinds.Whether intended for temporary or permanentcover, the transplanted skin does not only protectthe host bed from further trauma, but also pro-vides an important barrier to infection.
Thin split-thickness skin grafts have the best“take” and can be used under unfavorable condi-tions that would spell failure for thicker split-skingrafts or full-thickness grafts. On the minus side,thin STSG tend to shrink considerably, pigmentabnormally, and are very susceptible to trauma.9
In contrast, full-thickness grafts, being thicker bynature than STSG, require a well-vascularized re-cipient bed9 until graft perfusion has been reestab-lished. On the plus side, full-thickness skin doesnot contract upon grafting, resists trauma better,and generally looks more natural after healing.
Rudolph and Klein9 review the bio-logic events that take place in a skingraft and its bed. An ungrafted woundbed is essentially a healing wound,which if left alone will undergo the typi-cal processes of granulation, contrac-tion, and reepithelialization to seal itssurface.10 When a skin graft is placedon a wound bed, these processes arealtered by the presence of the graft.11
Marckmann12 studied biochemicalchanges in a skin graft after placementon a wound bed and noted similaritieswith normal skin in its response to physi-cal or chemical injury and aging. Thechanges in wound healing broughtabout by the skin graft can also be de-scribed as a general adaptation of con-
nective tissue to a diminished blood supply.12
EPIDERMIS
In the mid-1940s Medawar studied the behav-ior and fate of healing skin autografts.13-15 His find-ings are summarized as follows.
Histologic Aspects
During the first 4 days postgraft there is tremen-dous activity in the graft epithelium, which doublesin thickness, with crusting and scaling of the graftsurface. Three cellular processes may explain thisthickening: 1) swelling of the nuclei and cyto-plasm of epithelial cells; 2) epithelial cell migrationtoward the surface of the graft; and 3) acceleratedmitosis of follicular and glandular cells. By thethird day after grafting there is considerable mi-totic activity in the epidermis of a split-thicknessskin graft, whereas mitotic activity in full-thicknessskin grafts is much less common and may be to-tally absent—a reflection of their less-efficient earlycirculation.
Between the fourth and eighth days after graft-ing there is great proliferation and thickening ofthe graft epithelium associated with obviousdesquamation. Epithelial thickness may increaseup to sevenfold, with rapid cellular turnover. Atthe same time, the surface layer of epithelium ex-foliates and is replaced by upwardly migrating cellsof follicular epithelium at an accelerated rate. Thisheightened mitosis does not begin to regress until
Fig 1. Split-thickness skin grafts include a variable amount of dermis. Full-thickness grafts are taken with all the dermis. (Reprinted with permission fromGrabb WC: Basic Techniques of Plastic Surgery. In: Grabb WC and Smith JW:Plastic Surgery, 3rd Ed. Boston, Little Brown, 1979.)

SRPS Vol 9, No 1, 1999
3
after the first week postgrafting. By the end of thefourth week postgraft, the epidermal thickness hasreturned to its normal, pregraft state.
Histochemical Aspects
The RNA content of graft epithelial cells changeslittle in the first few days postgraft.16 By the fourthday postgraft, RNA content increases greatly inthe basal layers of the epithelium, paralleling thehyperactivity of epithelial cells. This hyperactivityreflects an acceleration of protein synthesis duringa period of rapid cellular replication. By the 10thpostgraft day, the RNA level returns to normal.16
Over the first 2 to 3 days, enzymatic activityprogressively decreases in split-thickness skin grafts,but as new blood vessels enter the dermis–epider-mis junction, the enzyme levels rebound.
DERMIS
Cellular Component
The source of fibroblasts in a skin graft remainsobscure.17 Early investigators believed that thesecells came from large mononuclear cells in theblood, while Grillo18 theorized that they originatedfrom local perivascular mesenchymal cells. What-ever their origin, most authors are convinced thatactive fibroblasts in a healing skin graft do notcome from indigenous fibrocytes.
Converse and Ballantyne19 studied cell viabilityin rat skin grafts by assaying levels of diphospho-pyridine nucleotide diaphorase, an indicator ofactive electron transport. The authors noted fall-ing fibrocyte numbers in the first 3 days after graft-ing. The remaining fibrocytes lay in two narrowlayers, one beneath the dermis–epidermis junc-tion and the other just above the host bed. Afterday 3 fibroblast-like cells began to appear, first inthe graft bed and later in the graft itself. By theseventh to eighth day postgraft, the fibroblast popu-lation and enzymatic activity were greater than innormal skin. After this early burst in fibroblasticactivity, however, both fibroblast numbers andenzyme levels resumed their normal, pregraft statesover the ensuing weeks.
Fibrous Component
Medawar13,14 stated that most of the collagen inan autograft persists through the 40th day after
grafting. Hinshaw, Miller, and Cramer,20,21 on theother hand, concluded that split-thickness and full-thickness skin autografts undergo considerablecollagen turnover. In their experiments the der-mal collagen became hyalinized by the third orfourth day postgraft, and by the seventh day all ofthe collagen was replaced by new small fibers.The replacement continued through the 21stpostgraft day, and by the end of the sixth weekpostgraft all the old dermal collagen had been com-pletely replaced. Interestingly, the rates of col-lagen turnover and epithelial hyperplasia peakedsimultaneously in the first 2–3 weeks postgraft.
Klein22,23 and Peacock24 used hydroxyprolineassays to determine the collagen content of graftedwounds. Hydroxyproline is an amino acid foundexclusively in collagen at a constant proportion of14%. Changes in hydroxyproline and monosac-charide content of grafted beds paralleled thoseof other healing wounds.25 Independent studiesby Hilgert26 and Marckmann27 confirmed these find-ings and documented plunging levels of hydrox-yproline soon after grafting. The hydroxyproline(and therefore, collagen) level eventually re-bounded and finally returned to the normal levelsof unwounded skin. Although Hilgert’s cycle lasted10 days and Marckmann’s lasted 14–21 days, it isnow well established that most of the collagen in agraft is ultimately replaced.
On the basis of studies involving tritiated pro-line-labeled mature collagen, Udenfriend28 andRudolph and Klein29 agreed that 85% of the origi-nal collagen in a graft is replaced within 5 monthspostgraft. The collagen turnover rate of grafts is 3to 4X faster than that of unwounded skin.30 Inaddition, although equal amounts of collagen arelost from full- and split-thickness grafts, STSGs re-place only half as much of their original collagenas do FTSGs of equal size.
Elastin fibers in the dermis account for the resil-ience of skin. While the elastin content of thedermis is small, the elastin turnover rate in a heal-ing graft is considerable, and most of the elastin ina graft is replaced within a short time. Elastin fiberintegrity is maintained through the third postgraftday, but by postgraft day 7 the fibers are short,stubby, and have begun to fragment.20 Elastindegeneration continues through the third post-graft week until new fibers can be seen begin-ning to grow at 4 to 6 weeks postgraft. Thisreplacement process is the same in full- and split-thickness skin grafts.

SRPS Vol 9, No 1, 1999
4
Extracellular Matrix
Far from simply supporting cells passively, theextracellular matrix (ECM) plays a vital role in cell-to-cell communication.31 Through specific arrange-ments of protein sequences within, the ECM influ-ences cellular behavior in adjacent tissues withregard to proliferation, differentiation, migration,and attachment.
The extracellular matrix in the skin consists oflarge insoluble proteins of fibroblast origin andsmaller soluble proteins produced either by fibro-blasts or keratinocytes. Both kinds of proteins ap-pear to be involved in directing the behavior ofkeratinocytes and in promoting appropriate com-munication between keratinocytes and fibroblasts.
Epithelial Appendages
The sweating capability of grafted skin is a func-tion of the number of sweat glands transplantedduring grafting and of the extent of sympatheticreinnervation to the graft. A skin graft will sweatmuch like its recipient site due to ingrowing sym-pathetic nerve fibers from the graft bed. Thus agraft that is placed on the abdomen will sweat inresponse to physical activity, whereas an identicalgraft placed on the palm will sweat in response toemotional stimuli.
Although both full- and split-thickness skin graftsdemonstrate sebaceous gland activity, thin split-thickness grafts do not contain functional seba-ceous glands and typically appear dry and brittleafter take.
Hair follicles are subjected to the same hyper-plastic stimuli as the rest of the graft. On thefourth day following grafting, the original hairsloughs off and the graft becomes hairless. Soonafter the graft follicles begin to produce new hair,and by the 14th postgraft day very fine, baby-likehair is seen growing out of the graft.13
Full-thickness skin grafts produce hair while split-thickness skin grafts produce little or no hair. Full-thickness skin grafts that take well grow normalhair in terms of orientation, pigmentation, and fol-licular clustering.14 Suboptimal graft revasculari-zation will damage the graft hair follicles and re-sult in decreased hair density. Similarly, whengraft take is interrupted for any reason, subsequenthair growth will be sparse, random, and lacking inpigment.15
In summary, unlike STSGs, full-thickness graftscontain sweat glands, sebaceous glands, and hairfollicles.8 Only full-thickness skin grafts, therefore,are capable of sweating, oil secretion, and hairgrowth.
GRAFT TAKE
As mentioned above, the large array of physi-ologic events usually seen in a healing skin woundare altered and modified by placement of a graft.The graft becomes incorporated in the host bedthrough the process of graft take. The success of agraft—its take—depends on the extent and speedat which vascular perfusion is restored to this para-sitic, ischemic tissue.
Given equal clinical and technical conditions,two qualities of a skin graft influence its fate. Thefirst determinant is the blood supply of the skinfrom which the graft was harvested. A graft takenfrom a highly vascular donor site will predictablyheal better than a graft taken from a poorly per-fused area. The second factor in graft take is themetabolic activity of the skin graft at the time ofapplication, which will dictate its tolerance to theinevitable period of ischemia.
Skin graft take occurs in three phases. The firstphase consists of serum imbibition and lasts 24 to48 hours. This is followed by an inosculatory phaseand a process of capillary ingrowth that occur es-sentially simultaneously, until generalized bloodflow has been established by the fifth or sixthpostop day.
Plasmatic Imbibition
The exact significance of plasmatic imbibitionto the healing of a skin graft is not clear. Hinshawand Miller20 and Pepper32 believed that plasmaticimbibition is nutritionally important. Others suchas Clemmesen,33,34 Converse,35,36 and Peer37,38 wereof the opinion that serum imbibition merely pre-vents the graft from drying out and keeps the graftvessels patent in the early postgraft period. Re-gardless of whose theory is correct, all authorsconcur in the following:
The graft is ischemic for an undeterminedperiod of time that varies according to thewound bed: 24 hours for a graft placed on abed that is already proliferative; 48 hours fora graft covering a fresh wound.

SRPS Vol 9, No 1, 1999
5
Grafts placed on poorly vascularized bedswill be ischemic for a longer time than thoseplaced on wounds with good blood supply.Exactly how long a graft will tolerate this is-chemic interval is not clear, but thick full-thick-ness grafts seem to tolerate ischemia for upto 3 days while thin full-thickness grafts sur-vive for up to 5 days.33,38 Split-thickness graftstake well even after 4 days of ischemia.38
Plasmatic imbibition allows a skin graft to sur-vive this immediate postgraft ischemic perioduntil such time as graft vasculature reestab-lishes itself.9
Grafts gain weight during the phase of plas-matic imbibition,34-36 adding as much as 40%to their pregraft weight through fluid move-ment from bed to graft.36 The origin of graftedema is believed to be the same as that ofinflammatory edema—ie, from disaggregationand depolymerization of proteoglycans, ac-cumulation of osmotically active metabolites,and increased vascular permeability.39-42
Inosculation and Capillary Ingrowth
At the end of 48 hours, a fine vascular networkis established in the fibrin layer between the graftand its recipient bed. Capillary buds from theblood vessels in the recipient bed make contactwith the graft vessels and open channels areformed. Blood flow is established and the skingraft becomes pink.
Revascularization
Three theories have been put forth to explainhow a skin graft is revascularized.
Connection of graft and host vessels. The firsttheory holds that after the inosculatory event, thedefinitive vasculature of the graft consists of theblood vessels originally present within the graft.According to this theory, circulation is restored ina graft via the original skin graft vessels by anasto-moses formed between the recipient bed and theskin graft through inosculation. Peer and Walker,37
Clemmesen,33,34 Haller and Billingham,43 and Birchand Branemark,39,40 among others, support this lineof thinking.
Clemmesen,34 working on a pig model, injectedIndia ink into the host vessels of the autograft. No
ink was seen within the graft in the first postgraftday, but on day 2 a number of graft vessels con-tained India ink, suggesting communication be-tween the host and graft vessels. After the secondday, many graft vessels contained India ink, indi-cating patent connections between vessels of thegraft and its bed. Initially a fine fibrin mesh linkedthe graft to the bed, but over the first 4 days thismeshwork became lined with endothelial cells andlinked up with the vessels of the graft.
Haller and Billingham43 reached a similar con-clusion in a study using the hamster cheek pouchmodel. They too noted that the pattern of vesselsin the healed graft was the same as the patternbefore grafting.
Formation of new vascular channels. The sec-ond theory of graft revascularization holds that thegraft is perfused through new vessels going fromthe recipient bed into the transplanted graft. Con-verse,19,36,44-46 Zarem,47 Ljungvist and Almgard,48 andWolff and Schellander49 espouse this theory.
Converse and Rapaport44 studied skin grafts inhumans and determined that after an early con-nection of graft and host vessels—the inosculatoryevent—, active invasion of the graft by host vesselsgives rise to the definitive vasculature of the skingraft.
On the basis of a later study involving diapho-rase in a rat model,19 Converse concluded thatdefinitive vasculature of the graft stemmed fromingrown vessels from the host bed. Degenerativechanges in the original graft vasculature were ap-parent in the first 4 days postgraft, as evidencedby progressive loss of diaphorase activity duringthis time. With subsequent vessel ingrowth therewas return of diaphorase activity.
Wolff and Schellander49 measured cellular en-zymes to evaluate return of circulation in porcineskin grafts. ATP activity correlated well with thepattern of new vessel ingrowth, leading the authorto conclude that the new graft vasculature con-sisted entirely of ingrown vessels.
Working with mice, Zarem et al47 theorized thatpreexisting graft vessels served only as nonviableconduits through which the endothelium of theingrowing vessels progressed. The rate of vesselingrowth was measured at approximately 5 mi-crons per hour. According to the authors, theoriginal graft vessels degenerated concomitantlyand at the same rate, leaving those vessels grow-

SRPS Vol 9, No 1, 1999
6
ing from the recipient bed as the definitive vascu-lature of the graft.
Combined old and new vessels. Smahel11 andTsukada50 proposed a third (and much less popu-lar) hypothesis of final graft revascularization: acompromise between the two above theories. Theauthors feel that circulation in a graft is reestab-lished in various ways; that is, in any graft old ves-sels may be recycled and new ones may grow tovariable degrees. These two pathways to restorecirculation to ischemic tissue may occur simulta-neously or as consecutive stages in the interactionbetween the graft and its bed.
There are two methods of skin graft revascu-larization: primary and secondary.
Primary revascularization. Under the scanningelectron microscope, it can be seen that no realcirculation to the graft exists for the first 6 to 7days postgrafting. Whatever flow there is withinthe graft is sluggish, varying in direction, with at-tendant pooling and pendulum-like movement.51
Clinically this manifests as cyanotic discolorationand is particularly noticeable in full-thickness skingrafts.19,39,44
In the normal course of events, circulation in askin graft is reestablished through vascular anasto-moses between budding neovessels from the bedand those already present in the graft (inoscula-tion). Blood enters the graft via these newly formedvascular connections and the graft turns pink. Apink color is generally considered a sign of prob-able graft survival, although the intensity of colora-tion does not allow any conclusions regarding thegraft’s circulatory status.
Inside the graft, the hemodynamic situation iscomplex. The old vessels of the graft are dilatedand denervated and some of the circulatory routesare severed during graft harvest. Blood vesselsfrom the recipient bed attach to both arteries andveins of the graft, yet all these connections areafferent with respect to the graft. Blood and tissuefluids moving into the graft are trapped there andunable to return to the bed because of inadequatereverse circulation.
Sometime between the fourth and seventh daysafter grafting, the newly formed vascular connec-tions differentiate into afferent and efferent ves-sels and other vessels retain their capillary-like char-
acter or simply disappear.52 At this point the propervascular system within the graft is reestablishedand blood flow is restored.
Secondary revascularization. When vascularconnections between the bed and the graft aredelayed, secondary revascularization occurs. Un-der normal graft conditions, the vasoactive agentdirecting the ingrowth of new blood vessels ceasesto function and capillary proliferation stops as goodblood flow is established via neovascularization.However, the longer a graft remains ischemic, thelonger the vasoactive substance remains in thetissue. As a result, great numbers of new capillar-ies grow into the graft and granulation tissue accu-mulates under the graft. This phenomenon isknown as secondary revascularization and itsmechanism is as follows.
Vascular connections between the graft bed andthe graft with attendant blood flow into the newlyformed vessels of the graft inhibit the formation ofcapillary buds. If the graft is not well applied to thebed and vascular connections are not establishedearly—eg, in the periphery of large grafts—, the in-hibiting effect of the vascular connection does nottake place. Within the graft itself the vessels maybe functionally deficient or the vascular ingrowthmay not reach the required level of biologic activ-ity for the inosculatory event. If these anastomosesfail to develop in time, the ischemic period is ex-tended and capillary proliferation in the bed con-tinues. Degenerative processes in the graft andexuberant granulation tissue in the host bed gohand in hand with prolonged ischemia. In sum-mary, if blood vessels reach the graft in time, thegraft will survive; if not, the graft will fail.
In the host bed, insufficient vascular prolifera-tion and wound contamination are the two com-mon causes of delayed inosculation. Anastomosesmay not form at the right time because of theincreased distance between the graft and its bedresulting from interposed necrotic material, a thickfibrin layer, hematoma, seroma, or air bubbles.
Grafts that heal by secondary intention aresmooth, fibrotic, tight, and have a slick, silverysheen on the surface, reflecting the large amountof cicatrix within the graft. Large grafts oftenheal both by primary and secondary revascular-ization, and certain areas show the typical ap-pearance of desquamation where the secondaryprocess occurs.

SRPS Vol 9, No 1, 1999
7
Histologically, the epidermis and papillary der-mis are destroyed by necrosis in the full-thicknessgraft that has healed by secondary revasculari-zation. The papillary dermis is replaced by a thinlayer of connective tissue which in turn is coveredby a flattened epidermis. The reticular dermis isnormal histologically, but beneath the graft thereis a layer of newly formed connective tissue thatinfiltrates the dermis, resulting in graft fibrosis.Hinshaw and Miller20 noted accelerated collagenturnover in pig autografts that had healed by sec-ondary revascularization.
Adherence
Adherence of the graft to its bed is the firstrequirement of skin graft take. A thin fibrin layerholds the graft to the bed and forms a barrier againstpotential infection.53
Two distinct phases of graft adherence occur.Phase 1 begins immediately after grafting and lastsabout 72 hours.54 During this time the graft re-mains adherent to the bed through the bondformed by the fibrin layer. Phase 2 coincides withthe onset of fibrovascular ingrowth and vascularanastomoses between the graft and the host.
Successful fixation of STSG to tangentially ex-cised wounds using fibrin glue is well docu-mented.55,56 Fibrin glue is manufactured from au-tologous plasma, eliminating the risk of transmis-sion of viral diseases. The material is strong, trans-parent, does not interfere with the healing pro-cess, does not promote wound infection, and ishomeostatic. Proponents of fibrin glue say that itimproves graft survival, reduces blood loss, speedsreconstruction by allowing large sheet graft cover-age, and produces better esthetic results.
Cederholm-Williams57 supports the use of fibringlue, emphasizing its many applications and supe-riority to bovine proteins. Dean, Nicholls, andWedderburn58 note the usefulness of fibrin glue inthrombocytopenic patients. Achauer and col-leagues,59 however, found no advantage to topicalfibrin glue in the healing of graft donor sites inburn patients.
A number of recent articles address new meth-ods of graft fixation. Himel et al,60,61 Sheridan andcoworkers,62 and Best and colleagues63 describethe use of disposable graft staplers to hold freshlyapplied skin grafts in place with absorbable staples.The advantages of these devices over standard
metal staples or bolstered sutures are said to in-clude speed of delivery, ease of use, reliable appli-cation, and no postoperative staple removal.
Skin grafts to the penis and scrotum are particu-larly difficult to immobilize and dress. Netscher,Marchi, and Wigoda64 suggest wrapping the graftarea with nonadherent gauze mesh over whichReston self-adhering foam is secured with staplesor sutures. The foam maintains penile length andprovides firm but gentle compression on the skingraft during the crucial first week. The authors citeease of application and removal, sterility, and ef-fectiveness in wound coverage as advantages ofthis method for grafted defects of the penis andscrotum.
Saltz and Bowles65 and Caldwell and colleagues66
also advocate the use of Reston foam applied overXeroform gauze for securing skin grafts in woundsof the shoulder and face, respectively.
Balakrishnan67 prefers Lyofoam, a semiperme-able, nonwoven polyurethane foam dressing forsimilar purposes. The foam is easy to apply di-rectly on the graft and, because it is biologicallyinert, does not interact with the wound. Its hydro-phobic outer surface is purported to retard bacte-rial colonization.
Johnson, Fleming, and Avery68 opt for a simple,versatile, and rapid technique consisting of staplesand latex foam dressing to secure skin grafts. Wolfand coworkers69 confirmed the effectiveness ofrubber foam with staple fixation in various pat-terns for providing even pressure distribution onskin grafts.
Smoot70 uses a Xeroform sandwich filled withmolded cotton balls that is stapled into place, whileAmir et al71 modify a cutoff disposable syringe toaffix the silk threads of their graft dressings. Chengand colleagues72 accomplish graft fixation with adisk cut from the bottom of an IV infusion bottle.Multiple radial slits are made along the perimeterfor tying the sutures holding the graft in place.
Other suggested fixation methods for grafts in-clude silicone rubber dressings73 and silicone gelsheets,74 rubber band stents,75 transparent gasbagtie-over dressings,76 Coban self-adherent wrap,77
thin hydrocolloid dressings,78 and assorted Silasticand foam dressings for grafts to the neck or hand.79-81
Schneider, Morykwas, and Argenta82 report us-ing a Vacuum-Assisted Closure (VAC) device tosecure grafts in difficult recipient sites. The VACpump prevents fluid collection beneath the graft

SRPS Vol 9, No 1, 1999
8
and ensures contact and even pressure distribu-tion between the graft and its bed, regardless oflocation or irregularity of surface.
GRAFT HEALING
Medawar13-15 described the clinical appearanceof healing grafts. Immediately after removal fromthe donor area the skin graft is white, but onceapplied to the recipient area it becomes pink overthe next few days. There is blanching on pressurewith prompt capillary refill. At first the graft sur-face is depressed below the level of the surround-ing skin, but by the 14th to 21st postgraft day itbecomes level with the surrounding surface.20
Histologically, lymphatic drainage is presentthrough connections between the graft and hostlymphatics by the fifth or sixth postgraft day, andsubsequently the graft loses weight until the pregraftweight level is attained by the ninth day.83 Col-lagen replacement in the graft begins by the sev-enth postgraft day and is complete in about 6weeks. The healing graft exhibits an abundance ofpolymorphonuclear lymphocytes and monocytesthat persist in the dermis for several weeks.
Vascular remodeling in the graft may take manymonths.84 Host vessel ingrowth is perpendicularto the dermis–epidermis junction, creating a char-acteristic vascular pattern in the graft. The newlyformed vessels are more numerous and showgreater arborization than those of normal skin.Other phenomena associated with healing of askin graft are discussed in detail below.
Contraction
A skin graft begins to shrink immediately afterbeing harvested from its donor site. Primary con-traction is passive and probably due to recoil ofthe dermal elastic fibers. A full-thickness graft losesabout 40% of its original area as a result of primarycontraction; a medium-thickness split-skin graftabout 20%; and a thin split-thickness graft onlyabout 10%. True Thiersch grafts do not undergoprimary contraction.
After transfer to a recipient site, the skin graftwill shrink as it heals in a process known as sec-ondary contraction. Full-thickness grafts tend toremain the same size (after primary contraction)and do not show secondary contraction. Split-thickness grafts, on the other hand, contract when-ever the circumstances allow. Unless split-thick-
ness skin grafts are fixed to underlying rigid struc-tures and cannot move, they will contract second-arily. Once wound contraction ends, full-thick-ness grafts are able to grow whereas split-thick-ness grafts tend to remain in a fixed, contractedstate and grow minimally, if at all.85,86
Wound contraction is a critical part of woundhealing and clinically useful because it reduceswound size. On the other hand, a contractedwound is often tight and immobile and there isdistortion of surrounding normal tissue. It is pos-sible to exercise some control on the degree ofcontraction that a wound will undergo by manipu-lating the thickness and proportion of dermis inthe graft used for repair. The inhibitory effect de-pends more on the percentage of dermis includedin the graft than in the overall thickness of thegraft: the greater the proportion of dermis includedin the graft, the more the graft will inhibit contrac-tion. Full-thickness grafts, therefore, prevent woundcontraction better than split-thickness grafts.85-87 Infact, thin full-thickness grafts inhibit wound con-traction better than split-thickness grafts of equalor greater thickness, and thick split-thickness graftsinhibit wound contraction better than thinones.8,88,89
Various hypotheses have been proposed to ex-plain the mechanism of this inhibition, including amass effect, cellular interactions between graft cellsand host bed, epidermal interaction, mechanicalrestriction, etc.90
Brown, Garner, and Young90 concluded that thecapacity of a skin graft to inhibit wound contrac-tion is directly proportional to the amount of struc-turally intact dermal collagen present in the skingraft. The rate of wound contraction is not af-fected by graft orientation, amount of epidermis,or noncollagenous protein. However, if theamount of intact dermal collagen is decreased oris structurally destroyed, then the graft’s ability toinhibit wound contraction is reduced.
Rudolph91,92 explains the interaction between agraft and its bed in terms of the lifecycle of amyofibroblast. Application of a skin graft appearsto check the stimulus of a wound to myofibroblastformation, function, or both.93 Split-thickness graftscause a rapid decline in the number of myofibro-blasts, and wounds contract less than comparablenongrafted sites. Full-thickness grafts trigger aneven faster decrease in myofibroblast population,and wounds show minimal contraction. In thishypothesis the lifecycle of myofibroblasts is accel-

SRPS Vol 9, No 1, 1999
9
erated rather than retarded, thereby reducingwound contraction.
Bertolami and Donoff88,89 studied the effect ofdermis on the actinomycin content of granulatingwounds and suggest that the mechanism of woundcontraction is not simply the result of myofibroblastactivity;93 the active role of collagen cannot beignored. Substances that inhibit wound contrac-tion also inhibit prolyl hydroxylase activity (an indi-cator of collagen synthesis). Lower levels of thisenzyme beneath a full-thickness graft may reflectdecreased collagen synthesis, which in turn maybe involved in preventing wound contraction.88
Oliver and associates94,95 demonstrated the im-portance of an inactive collagen matrix in inhibit-ing wound contraction. The collagen matrix wasprepared for grafting by adding azide to destroythe cells and trypsin to remove noncollagenousprotein. These grafts, cell-free and noncollagenous-protein-free, resist wound contraction as well asfull-thickness skin grafts, suggesting that dermal cellsand noncollagenous proteins are not part of theinhibitory process. Grafts free of dermal cells butpossessing a collagen matrix in fact behave muchlike full-thickness grafts. It may be possible, there-fore, to store nonantigenic dermal substitutes pro-duced from banked cadaver skin or xenogeneicsources by adding trypsin or azide to removenoncollagenous protein and cells respectively. Thiswould increase dramatically the clinical availabilityof substitute dermis as a potential source of grafts.
Reinnervation
Nerves grow into skin grafts from the woundmargins and the graft bed.96 The timing of neuralinvasion and the disposition of nerves within askin graft vary with graft thickness and recipientsite. Human skin grafts begin to show sensoryrecovery at 4 to 5 weeks postgrafting, but occa-sionally sensation is delayed for up to 5 months.The return of normal sensation is usually completeby 12 to 24 months. The extent of reinnervationdepends on how accessible the neurilemmalsheaths are to the invading nerve fibers—ie, mostaccessible in full-thickness grafts and least acces-sible in thin split-thickness grafts.
Skin grafts are initially hyperalgesic and thenslowly regain normal sensation.8 If skin graft heal-ing is uneventful, the results of two-point discrimi-nation testing will be very close to those of normalskin. Other sensations do not recover so well.
Waris and associates96 measured the thermal sen-sitivity of 22 split skin grafts transplanted 1-4 yearsearlier. Cold sensitivity was present in 14, warmthin 6, and heat-pain in 8 grafts. If the warmth sensi-bility had recovered, the threhold was lower thanfor cold. Seven grafts showed no thermal sensibil-ity at all.
Haro and colleagues97 confirmed poor returnof sensitivity in grafts using immunohistochemicalmethods. Grafts less than 7 months old showedno sensitivity whatsoever, and pain sensation haddeveloped only in the 15-month-old grafts. Al-though deep and superficial nerve plexuses regen-erated, no sensory corpuscles were detected ingrafted skin at any time.
Stella et al98 independently verified these find-ings and speculate that the failure of regenerationof sensory corpuscles may be related to the de-generation of periaxonal corpuscular elements.
Ponten99 stated that grafts assume the sensorypattern of the host tissue, but Adeymo andWyburn100 and later Fitzgerald, Martin, andPaletta101 noted that nerves entering the graft fol-low the evacuated neurilemmal sheaths and rees-tablish the innervation pattern of the donor skin.
Weis-Becker and coworkers102 note better rein-nervation of split skin grafts placed on intact musclefascia than if the fascia had been removed. Sen-sory functions on grafted skin were generallyreduced.
Pigmentation
Immediately after harvesting, a skin graftblanches from circulatory interruption. The con-sequent loss of melanoblast content causes pro-found alteration in the ratio of pigment-producingto nonpigment-producing cells in the graft.103 Af-ter transplantation and graft revascularization, thereis inflow of erythrocytes and the normal equilib-rium of melanocyte population is restored. Thegraft resumes a pink coloration which over timefades to a basically normal skin tone. Mir y Mir104
reviews melanogenesis, its peripheral nervous sys-tem control, the hyperpigmentation state that fol-lows cutaneous grafting, and the effects of ultra-violet radiation on the skin.
Skin grafts change color during healing.11 Graftsharvested from the abdomen, buttocks, and thighbecome darker as they heal, while grafts takenfrom the palm tend to lighten. Grafts taken frombrunettes progressively darken, while those from

SRPS Vol 9, No 1, 1999
10
blondes usually lighten. Full-thickness grafts fromthe eyelid, postauricular and supraclavicular areasare usually good color match for the face, althoughthey may remain red for many months. In general,grafts taken from below the clavicle assume a yel-lowish-brown hue, while grafts taken from abovethe clavicle provide a better color match for facialskin. Thin split-thickness skin grafts from the samedonor site are usually darker than thick ones.99
The best treatment for hyperpigmented grafts isdermabrasion. For dermabrasion to be effective,however, it must be done at the appropriate time.If it is performed too soon after skin graft, theblanching will not last and the dark pigment willreappear. Best results are seen when dermabra-sion follows biologic reinnervation of the graft.Generally, the later the dermabrasion is done aftergrafting, the more effective it is in removing un-wanted pigment.
Skin depigmentation states and their treatmentare reviewed by Taki et al.105 Vitiligo, senile leuko-derma, dyschromatosis symmetrica hereditaria, andsecond- or third-degree burns can produce signifi-cant cosmetic deformity, particularly in dark-skinned patients. Corticosteroids and oral psoralenmay, with exposure to sunlight, be successful inthe treatment of vitiligo provided that dopa-posi-tive melanocytes are present in the skin.106 Burnsthat invade the dermis (second-degree and third-degree) decrease the number of dopa-positivemelanocytes, so that appropriate treatment neces-sitates removal of the depigmented skin and re-placement by very thin split-thickness grafts of nor-mal color. This protocol is also successful in treat-ing leukoderma.
A number of authors report successful repig-mentation in leukoderma or vitiligo after treatmentwith ultra-thin, melanocyte-containing epidermalsheet grafts107-114 or in-vitro cultured melanocytes.115
Hosokawa and colleagues116 report a novel methodof tattoo elimination in which the epidermis of thetattooed skin is replaced after chemically remov-ing the dermis, which contained tattoo pigments.Wound healing time was much shorter than fortypical skin grafts.
OVERGRAFTING
Dermal overgrafting consists of applying a split-thickness skin graft to a recipient bed of dermis ordenuded scar tissue.117 Overgrafting preserves
subcutaneous tissues, is a relatively simple proce-dure, and the tissue consequences of graft failureare minimal. Rees and Casson118 offer technicaldetails of skin removal and bed preparation andlist the best donor sites. Their indications forovergrafting are as follows:
unstable, depressed, corrugated, or hyper-trophic scars
unstable or hyperpigmented skin grafts
large pigmented nevi
radiation damage
tattoos
Pigmented lesions should be excised deepenough to remove all the pigment before the graftis applied. A potential complication of the tech-nique is the formation of cysts and granulomasfrom retained epithelial remnants.118
GRAFT EXPANSION
Because a wound is reepithelialized from theedges toward the center, the perimeter of the graftis the only part that contributes to the epithelializa-tion process. An expanded graft presents a largercumulative diameter through which epithelial out-growth can proceed. With graft expansion, largerareas can be covered with smaller sections of skin.
Various ways to expand skin for grafting havebeen described. These include pinch grafts,119 re-lay transplantation,120 meshing,121-124 Meek islandgrafts,125 and microskin grafts.126-131 More uncon-ventional is the Chinese technique of intermin-gling autografts and allografts.132,133
A pinch graft breaks up a whole graft of skininto tiny pieces to increase the edge area. Pinchgrafts are reported to be effective in treating smallto medium-sized venous leg ulcers,134,135 radioder-matitis, pressure sores, and small burns.119
Relay transplantation consists of cutting a graftinto strips 3–6 mm wide and 5–10 mm apart.When the epithelial growth becomes clinicallyobvious (5 to 7 days later), the original strips areremoved and transplanted, leaving the epithelialexplants in place. This process may be repeatedup to 4 times.120
Meshing is the term used for cutting slits into asheet graft and stretching it open prior to trans-plantation. Meshed grafts have a number of ad-vantages over sheet grafts: (1) meshed grafts will

SRPS Vol 9, No 1, 1999
11
cover a larger area with less morbidity to the pa-tient than nonmeshed grafts; (2) the contour of themeshed graft can be adapted to fit in a regularrecipient bed; (3) blood and exudate can drainfreely through the interstices of a meshed graft;(4) in the event of localized bacterial contamina-tion, only a small area of meshed graft will be jeop-ardized; (5) a meshed graft offers multiple areas ofpotential reepithelialization.121-123 The foremost dis-advantages of meshed grafts are the considerablesurface area that must heal by secondary intentionand the less-than-ideal cosmetic result. Davison etal122 solved these problems by using a small ratioof expansion—1:1.5—and pulling the graft length-wise to narrow the skin perforations to slits beforetransplantation.
Richard and colleagues124 compared the Tannerand Bioplasty skin graft meshing systems in termsof their respective expansion ratios and predictedversus actual expansion. Both systems deliveredapproximately 50% of the anticipated skin expan-sion, leading the authors to recommend harvest-ing skin grafts larger than needed to compensatefor the eventual shortage.
Kirsner and and associates136 analyzed their re-sults with meshed grafts in the treatment of recalci-trant leg ulcers and concluded that meshed STSGsare safe and effective therapy.
The Meek technique of graft expansion consistsof using a special dermatome and prefolded gauzesto expand autograft surfaces from small pieces ofsplit skin graft.125 The expansion ratio obtainedwith the Meek technique is almost 1:9. In con-trast, the expansion ratio of allograft meshed withthe Zimmer II dermatome set at 1:6 was measuredas 1:4. The Meek technique is a useful alternativeto meshed grafts when donor sites are limited. Theresultant expanded island grafts are particularlysuited for transplantation onto granulating woundsthat present poor grafting conditions.
The technique of microskin grafting in whichsheet grafts are minced using a Tanner-Vandeputdermatome has been described by Zhang,126,127
Lai,128 Lin,129,130 and Vandeput and Tanner,131 all ofwhom report successful coverage of burn wounds.The expansion ratio achieved is 1:10 and graft takeis said to be excellent even under poor graftingconditions.
Intermingled transplantation of autograft withallograft has been practiced successfully in Chinasince at least 1973,132,133 primarily in the treatment
of large burns. Yeh and colleagues137 comparedthis technique with the microskin method in a ratmodel and noted significantly less scar contrac-ture associated with the Chinese method. Otherhealing parameters were similar between the twogroups.
GRAFT SURVIVAL/FAILURE
A meticulous surgical technique contributesgreatly to the survival of a skin graft. Particularattention should be paid to ensuring
atraumatic graft handling
a well-vascularized, scar-free bed
careful hemostasis and removal of accumu-lated blood before dressing the wound
postoperative immobilization of the graft re-cipient site
use of a tourniquet during graft harvest andtransfer
no proximal constricting bandages
Flowers138 reviews the usual complications as-sociated with graft failure and recommends stepsto avoid them. The graft bed should be as clean aspossible, free of dead tissue, and have an appro-priate substrate (eg, bone should have periosteum,tendon should have peritenon). A clean area withendothelium is all that is required in the bed of asuccessful skin graft.
The most common cause of autologous skingraft failure is hematoma. The clot isolates theundersurface of the graft from the endothelial budsof the recipient site so that revascularization can-not take place.138
The second most common cause of graft loss isinfection. Infection can be avoided by carefullypreparing the wound bed, using quilting sutures,meshing or pie-crusting the graft surface to allowfree egress of subjacent fluids, and applying wetsaline dressings that are changed every 4 hours.138
Fluid beneath the graft can also cause graft ne-crosis. Areas rich in lymphatics such as supraclav-icular, inguinal, and axillary regions are particularlyprone to develop seromas. Atraumatic tissue han-dling, cauterization of lymphatic vessels, limiteduse of electrocautery in the graft bed, and a lightpressure dressing minimize the risk of fluid accu-mulation under the graft.138
Excessive pressure on a fresh graft may alsocause it to die. The applied pressure should never

SRPS Vol 9, No 1, 1999
12
exceed 30 mmHg. Other causes of graft failureinclude gravitational dependency, movement ofthe area, arterial insufficiency, venous congestion,and lymphatic stasis.
Teh139 studied 21 patients with stasis ulcers inan attempt to pinpoint the causes of graft failure.Wound exudates were assayed for fibrin degrada-tion products, fibrinogen, available plasminogen,and active plasmin. All wounds showed granula-tion tissue and were classified as clean or dirty.Clean wounds had low bacterial counts andshowed no detectable plasmin activity. Dirtywounds had high bacterial counts and increasedlevels of active plasmin. High plasmin and pro-teolytic enzyme activity was generally seen inwounds contaminated with beta-hemolytic strep-tococci and various species of Pseudomonas. Thepresence of fibrin under autografts was associatedwith success in 17 of 21 ulcers, and the absence offibrin was associated with graft failure. This findingsuggested to the author that dissolution of fibrinby plasmin and proteolytic enzymes is the prob-able mechanism in graft failure secondary to mi-croorganisms. In conclusion, a grafted wound isrendered sterile through the blocking action offibrin in the interface between the bed and thegraft. Fibrin plays a central role in graft survivaland is responsible for the antibacterial characterof adherent dressings and autografts. This bacte-riostatic effect of grafts has proved invaluable inthe management of large burns.139
Hill140 recommends a number of measures toenhance the survival of full-thickness grafts. Be-cause streptococci produce streptokinase andother enzymes that break down the fibrin clot anddecrease adherence of the graft to its bed, he pro-poses the administration of low-dose erythromy-cin for the first 5 days after grafting to combatpotential strep and staph colonization. Patientsshould also take vitamin C and zinc for a week to10 days to promote healing and should abstainfrom using alcohol for at least 2 days before and 5days after sugery.140 Ethanol in the bloodstreamhas been shown to decrease the initial phase ofwound healing (the PMN clean-up phase) and canresult in infection and decreased graft adherence.
Wolfort and colleagues,141 working with rabbits,found that epinephrine added to local anestheticsolutions decreased inosculation in full-thicknessgrafts but had no effect on ultimate survival ofsplit-thickness skin grafts. Subsequently Fazio andZitelli142 assessed the clinical effects of epineph-
rine in local anesthesia of the donor site. Theauthors found only an increased risk of graft com-plications at 1 week and no effect on the 6-weekcosmetic outcome. They do not recommend us-ing plain lidocaine for harvesting full-thickness graftsunless the vascular supply of the donor area iscompromised.
Robson and Krizek143 predict skin graft survivalon the basis of successful homograft take prior toautografting. Perry144 notes a direct correlationbetween skin graft survival and bacterial counts of<105 in the recipient bed.
DONOR SITE MANAGEMENT
Open Wound
The open-wound technique of donor-site man-agement is associated with prolonged healing time,more pain, and higher risk of complications. Mostauthors recommend covering the donor area inskin grafting as protection against trauma and in-fection.
Allen and associates145 obtained bacterial countsof wounds left open to granulate and of woundscovered by skin dressings. They found 12.5% ofthe open wounds were sterile but all the dressedwounds showed some microbial flora. Autograftsand allografts gave similar results. When antibiot-ics were added, however, there was a dramaticdecrease in bacterial colonization of the wounds,leading the authors to conclude that it was theantibiotic, not the dressing, that had a sterilizinginfluence. In other words, skin grafts have no in-trinsic bactericidal properties.146
Biologic Dressings
Autografts
Feldman147 recommends returning unused skinto the donor site as an autologous biologic dress-ing. He feels this is an effective and logical proce-dure from the standpoint of wound healing, tissueconservation, and expense.
Allografts
Traditionally, cadaver allografts have been themethod of choice for resurfacing large denudedareas. They serve as temporary wound covers,

SRPS Vol 9, No 1, 1999
13
reduce pain and fever, restore function, increaseappetite, control fluid loss, and promote woundhealing. As they revascularize, they form a barrieragainst bacterial invasion and prevent further lossof water, electrolytes, and protein from the wound.Allografts decrease bacterial counts of underlyingtissues, facilitating future grafting by promoting asterile wound bed.148
Allografts preserved in various ways have beenused successfully for decades. Hoekstra, Kreis,and du Pont149 trace the history of preservationtechnologies at the Euro Skin Bank in Holland sinceits founding in 1976. Initially allografts were treatedwith 0.5% glutaraldehyde and stored at 4ºC, butthis led to early deterioration characterized by epi-dermolysis. In 1983 the benefits of glycolizationbecame evident150 and henceforth cadaver al-lografts have been treated with glycerol beforefreezing.149-151 Modern cryopreservation tech-niques use 30% glycerol or 5% DMSO as cryo-protective agents. The graft is gas sterilized, dried,frozen, and stored in plastic containers for use upto 4 years later. Reconstitution involves 15 to 20minutes of agitated washout with normal salinesolution at room temperature.
Studies on the immunogenicity of glycerol-pre-served donor skin and its virucidal effect are en-couraging.152,153 Pirnay and colleagues154 reviewedthe literature for evidence of the presence of HIVin human skin and the possible transmission ofHIV by transplantation of human allograft skin.Reverse transcriptase polymerase chain reaction(RT-PCR) detection of viral RNA in plasma is themost sensitive HIV screening test, but is complex,expensive, and not very reliable. Even thoughavascular tissue processing can inactivate HIV,155,156
at the present time “it is unknown whether al-lograft processing can inactivate HIV without af-fecting the functional integrity of the allograft.”157,158
Glycerol-treated, cryopreserved allografts havea number of applications, including scald burns inchildren,159,160 for coverage of extensive burnwounds in children and adults,161 and, combinedwith allogeneic cultured epithelial grafts, for thetreatment of deep burns down to muscle fascia orfat.162,163 The major drawback of glycerol-preservedallografts is their expense—approximately $5 persquare inch for the final product.
As discussed above, Chinese investigators havesuccessfully used combinations of allografts andautografts for coverage of open wounds.132-134 Theautograft is cut into small pieces and placed in the
slits of meshed allografts or is laid down in alternat-ing strips of auto- and allograft. As rejection un-folds, epidermal cells in the autograft gradually re-place the allograft.164
The host-immune mechanism can also be ma-nipulated to prolong the interval from allograftapplication to rejection. Immunosuppressiveagents include alloantiserum,165-168 azothiaprine,148
antithymocyte globulin,148 and cyclosporine.148,169
Cyclosporine extends graft viability by suppress-ing T lymphocytes.169 First-set rejection is preventedby inhibiting donor-specific T cells, but second-setrejection proceeds in normal fashion, and futuregrafts from another donor are rejected.170
The techniques of intermingling grafts andimmunosuppresion extend the period that al-lografts can be left in contact with open wounds,allowing more time for regeneration of autogenousdonor sites and decreasing the amount of allograftrequired for cover. Kolenik and Leffell171 reportusing cryopreserved skin allografts followingMohs’s chemosurgery to provide continuouswound coverage until full-thickness grafting couldbe performed.
Xenografts
Xenografts (collagen–elastin prostheses) adhereto a wound bed via fibrin bonding, which is afunction of the collagen content of the dermis andis not related to viability. The advantages of xe-nografts are relatively low cost, ready availability,easy storage, and easy sterilization. Disadvantagesare lack of antimicrobial activity, no proof thatthey promote reepithelialization, potential for ab-sorption of toxic breakdown products, and poorperformance with respect to healing time and painwhen measured against other donor site dressings.
Csönge and colleagues172 preserve cadaver al-lograft and xenograft skin at their Tissue Bank inBudapest using a combination of 1000 mg/Lceftazidime, 1000 mg/L ampicillin, and 80 mg/Lamphotericin. To date there has not been a graft-originated infection in many burned patientstreated with this skin. Nevertheless, most authorstoday condemn the use of xenograft dressings inmodern clinical practice.
Amnion
Amnion is composed of an inner membraneand an outer membrane. The inner membrane isthe amnion proper and the outer membrane is the

SRPS Vol 9, No 1, 1999
14
chorion. The extraembryonic fetal membraneshave a mesenchymal surface in addition to theirepithelial (amniotic) and decidual (chorionic) sur-faces. These terms are used to describe how themembrane is placed on the wound.
The amnion has been used successfully in thetemporary dressings of leg ulcers, contaminatedor infected raw surfaces such as burns, postvulvec-tomy defects, gastroschisis, and pilonidal cyst si-nuses. Amniotic membranes are less expensivethan and superior to pig skin as biologic dressings.Amnion is more effective than human skin in de-creasing bacterial counts in burn wounds.173 Am-nion can be sterilized and stored at room tem-perature for up to 9 months after delivery.174 Theamniotic membranes effectively reduce pain, de-crease fluid and protein loss, and protect raw sur-faces until a permanent cover is available.
Neovascularization of amnion does not occur,and the membrane can be removed in 7 to 10days as the wound begins to close painlessly.However, amnion does not promote healing andappears to potentiate increased hyperemic reac-tions and scarring.175 In summary, amnion is inex-pensive, convenient, ubiquitous, and biologicallyeffective.176
Synthetic Materials
Feldman147 reviewed methods for dressing thedonor site of a skin graft (Table 1).
Synthetic wound dressings can be semiopen,semiocclusive, or occlusive. Semiopen dressingsinclude Xeroform, Biobrane, and fine mesh gauzeimpregnated with Scarlet Red or Vaseline. Semi-occlusive dressings include Op-Site, Tegaderm, andDuoDERM. Because they are bacteria- and liquidimpermeable, fluid tends to collect under thesedressings and should be drained frequently.
Feldman and colleagues177 evaluated the effec-tiveness of various donor site dressings in 30 pa-tients with respect to healing, pain, infection, andexpense. Xeroform was judged to be better thanBiobrane, with an average healing time of 10.46days, no infections, and an average cost per pa-tient of $1.16. The healing time for Biobrane was19 days and for DuoDERM was 15.3 days.Biobrane was more comfortable than Xeroformbut had 29% more infections and cost $102.50per patient.
In another study,178 donor site wounds dressedwith Op-Site and Tegaderm showed rapid, rela-
tively painless healing and low infection rates.These are expensive materials, however, particu-larly in dressing large donor areas, and must bechanged frequently due to fluid collection.
Brady179 compared Op-Site, Vaseline gauze,Jelonet, Scarlet Red, and exposure in terms of heal-ing time, pain, infection, and cost. Pig skin wasalso included in the study initially but was sooneliminated because of the incidence of Pseudomo-nas infection and hypertrophic scarring. Woundsdressed with Jelonet healed quickly, followedclosely by Vaseline gauze. The interval to healingwas longest with the open method. Op-Site wasthe most comfortable dressing according to pa-tients, but also the most expensive. Vaseline gauzewas second only to exposure in low cost. Recom-mendations from the authors were Op-Site orJelonet for dressing small donor areas and Vaselinegauze for large wounds.
Barnett and coworkers180 compared syntheticadhesive, moisture-vapor-permeable and fine meshgauze dressings for STSG donor sites with respectto pain, rate of healing, adherence, and infection.Op-Site and Tegaderm promoted fast healing witha mean of 6.8 days from graft to closure and werebasically painless. Wounds covered with fine meshgauze healed in 10.5 days but were 3X as painful.
Zapata-Sirvent181 compared Biobrane and Scar-let Red and found Biobrane was better for control-ling pain and exudate accumulation and had shorter
Table 1 Techniques for dressing skin graft donor sites
Semiopen Fine mesh gauzeScarlet RedVaseline GauzeXeroform
Biobrane
Occlusive Duoderm
Semiocclusive Op-SiteTegaderm
Biologic AutograftExcess skin graft
AllograftHuman cadaver skin
XenograftPigskin
Amniotic membraneCultured keratinocyte grafts
(Annotated from Feldman DL: Which dressing for split-thick-ness skin graft donor sites? Ann Plast Surg 27:288, 1991. Withpermission.)

SRPS Vol 9, No 1, 1999
15
healing times. Tavis et al182 agree that Biobranereduces pain, limits infection and desiccation, andoptimizes healing times, though it is considerablymore expensive.
Poulsen and colleagues183 found conventionalimpregnated gauze (Jelonet) superior to semiper-meable polyurethane film (Op-Site) in the treat-ment of outpatient partial-thickness burns, both interms of speed of healing (7 vs 10 d) and residualscars (8% vs 21%).
Lawrence and Blake184 as well as Porter185 evalu-ated Kaltostat, a calcium alginate dressing, in thehealing of split-thickness skin graft donor sites. Therate of epithelialization, pain, and convenience ofuse were measured and compared with those inpatients treated with Scarlet Red and DuoDERM,respectively. The authors documented slower heal-ing times with Kaltostat (15.5 d) than with eitherScarlet Red (10 d) or hydrocolloid (10 d). On theother hand, the alginate was easier to use andseemed to lend itself well to outpatient treatment.185
Dressings that provide a moist environment forthe wound speed the rate of healing. Nemeth etal186 noted faster healing by nearly 4 days and 6Xless pain in shaved biopsy sites treated withDuoDERM than those managed with conventionaltherapy consisting of hydrogen peroxide cleans-ing, bacitracin ointment, and bandaids.
Sawada et al187 reported prompt epithelializa-tion (average 7 days), no infection, and little painin donor site wounds treated with silicone gel sheetcontaining ofloxacin. Owen and Dye188 found thattopical application of 2% lignocaine gel to the skingraft donor sites controlled discomfort during thefirst week of grafting and did not impair healing.Alvi and colleagues189 attest to the safety of topicalanesthetic agents in gel form to provide relief atsplit skin donor sites.
SKIN SUBSTITUTES
Unlike temporary wound dressings, skin substi-tutes are designed to be left in place for long peri-ods of time. Fifteen years ago Pruitt and Levine190
listed attributes of the ideal skin substitute, whichare still current today:
little or no antigenicity
tissue compatibility
lack of toxicity, either local or systemic
permeability to water vapor just like normal skin
impenetrability to microorganisms
rapid and persistent adherence to a woundsurface
porosity for ingrowth of fibrovascular tissuefrom the wound bed
malleability to conform to an irregular woundsurface
elasticity for motion of underlying tissues
structural stability to resist linear and shearstresses
a smooth surface to discourage bacterial pro-liferation
sufficient tensile strength to resist fragmenta-tion
biodegradability
low cost
ease of storage
indefinite shelf life
Biological
Autologous skin substitutes basically combineallogeneic fibroblasts with collagen to form aneodermal matrix to which is added a suspensionof autologous epidermal cells.191 In early clinicaltrials these skin equivalents appeared to heal welland approximate the color of normal skin some 8months after grafting. At 18 months postgraft therewas no evidence of either fragility nor hypertrophicscar.
Other successful reports by Harriger et al,192
Maruguchi et al,193 and Beumer and colleagues194
attest to the growing popularity of the technique.Although slightly different from each other, theseskin equivalents are basically a composite of bo-vine type I collagen-containing sponge neodermiswith a suspension of cultured keratinocytes as theneoepidermis. Ghosh and associates195 reviewvarious techniques for the preparation of humanskin composites based on deepidermized acellu-lar dermal matrix, epidermal keratinocytes, anddermal fibroblasts.
In 1975 Rheinwald and Green5 pioneered amethod for growing human epidermal cells in vitro.Four years later Green et al,6 seeking to make aproduct suitable for grafting, perfected the tech-nique of cultured epithelial autografts. Subse-quently O’Connor and Green196 reported the clini-cal application of epithelial cell cultures in burn

SRPS Vol 9, No 1, 1999
16
patients, and since that time cultured epithelialautografts (CEA) have been used to solve a num-ber of clinical problems.
The tissue-culture technique is essentially simple.6
Skin biopsies are taken and the epithelial cells areseparated from the dermis by means of trypsin.Lethally-irradiated mouse fibroblasts are used as asurface on which to place the keratinocytes forgrowth. The cells are incubated for 10 to 14 daysin bovine fetal serum, at which time they are di-vided. A second generation of grafts is then grownin a similar medium for an additional 2 to 2.5 weeks,and by the end of 4 weeks a 1.5-cm skin biopsyhas grown into enough skin to cover a wound1.7 m2.
Phillips et al197 serially biopsied the cultured epi-dermal grafts which were applied to leg ulcers tostudy their histologic appearance. Ten days post-graft there was compact hyperkeratosis, a normal-looking epidermis, and a flat dermis–epidermisjunction. At 6 months postgraft the area lookedvery similar to hyperproliferative skin, with a basket-weave appearance of the stratum corneum. Thedermis–epidermis junction continued to be flat.
Teepe et al198 continued this line of work for aperiod of almost 5 years. The authors confirmedthe results of previous studies, namely that the cul-tured epidermis remains in a hyperproliferative statefor a long time after grafting. This unchecked pro-liferation could be the result of an absent modulat-ing dermal factor. Keratinocyte maturation follow-ing cultured autografts does not return to normalfor at least 4.5 years after healing of full-thicknessburn wounds.
Cultured keratinocyte grafts lack Langerhans cellsand T lymphocytes, thus it is felt that allogeneicepidermal grafts were immunologically protectedand could be used whenever the situation de-manded early wound coverage and could not with-stand the obligatory 3 to 4 week delay for culturedepithelial autograft. Phillips199 demonstrated thatcultured allogeneic epithelial grafts do not take inthe traditional sense, but are rapidly replaced byhost epithelium. In about 6 months after grafting,all the keratinocytes in the allogeneic grafts havebeen replaced by keratinocytes of recipient origin,so that by this time the allograft for all intents andpurposes has become an autograft. Other authorsconfirm these observations.200-204
Tomson and coworkers205 note improved in-vitrogeneration of epithelial cells harvested from theoral mucosa. Keratinocytes obtained from this
source replicate at a much faster rate than keratin-ocytes from normal biopsied skin elsewhere.
At the present time, cultured epidermal graftsare being used extensively in the treatment of burns,either alone,199-204,206-217 in combination with allo-geneic skin transplants,218,219 or as composite graftsof CEA and bipolymer.192 Cultured epithelial cellsto be used for grafting have an expansion capabil-ity of 10,000X the original surface area.206,220,221
When cultured cells and allografts are combined,they tend to be more stable than either of thecomponents alone, yet many prefer to use CEAalone on large total body surface area burns.222,223
In addition to their usefulness in massive burnsand chronic skin ulcers, cultured keratinocytes havebeen shown to be effective in treating keloids,224
vitiligo,208 epidermolysis bullosa,225 idiopathic pyo-derma gangrenosum,226 bullous pemphigoid,227 af-ter excision of giant hairy nevi,228 in postoperativeotorrhea from failure of epithelialization of openmastoid cavities,208,229 to cover skin graft donorsites,230 and in a number of other conditions.
One of the most popular applications of CEApreparations has been in the treatment of chronicleg ulcers.208,212,224,231-235 In terms of graft take,overall coverage, healing time, and cosmetic re-sult, CEAs compare favorably with standard punchgrafts. Green208 explains the effectiveness of CEAsin chronic ulcers as follows: Keratinocytes synthe-size a large number of polypeptide growth fac-tors, among them transforming growth factor al-pha (TGF-α), which promotes proliferation andmigration of the keratinocytes in a biofeedbackmechanism that stimulates growth of the cell whichproduces it.
Many burn centers continue to use culturedepidermal autografts. The following conclusionsregarding grafts of cultured keratinocytes derivefrom their combined reported experiences:
Tissue cultured grafts are commercially avail-able.
CEAs are very expensive: a 2 x 2 inch graftcost approximately $550 in 1996.
Sheets of cultured keratinocytes are very frag-ile and must be handled with extreme care.
CEAs require well vascularized beds.
Once the CEA takes, the cells will spread pe-ripherally to join other grafts or surroundingskin.

SRPS Vol 9, No 1, 1999
17
CEAs are extremely sensitive to infection, tol-erating maximum bacterial counts of 102 to103/cm3 (compared with 104–105/cm3 forstandard STSG).207
Synthetic
Synthetic skin substitutes may be solid or micro-porous, slitted or smooth, adherent or nonadher-ent, and unilaminar or bilaminar. Unilaminar mem-branes are of one of three types: hydrogels, hy-drocolloid dressings, or vapor-permeable mem-branes.236 These materials effectively debride thewound, absorb fluids, decrease bacterial counts,and stimulate granulation tissue growth.237,238 Theyseem to be the most effective in accelerating thehealing of partial-thickness wounds239 and do notprovide any mechanical protection. Bilaminar skinsubstitutes consist either of totally synthetic, bio-logically inert materials or collagen–synthetic com-posites.
Totally synthetic. In 1975 Levine et al240 deviseda totally synthetic, biologically inert bilaminar mem-brane for use as a skin substitute. The material ismade up of an inner layer or “dermis” which isnylon fabric, while the “epidermis” consists ofpolytetrafluoroethylene (PTFE). At its thinnest pointthe material is 0.635 mm thick. The PTFE is 1 milthick and has a 0.1 mcm pores. The membraneattaches to the bed of an excised wound within 5days, and in about 10 days fibrovascular tissue hasinvaded two-thirds into the dermis. The membraneallows passage of water vapor, protects the hostfrom infection, and increases patient survival inburns greater than 60% total body surface area.Clinically the membrane was applied to patientswith 30% to 75% TBSA burns and left in place for2 to 3 days, at which time it was removed and thewounds autografted. (By definition, then, the ma-terial qualifies as a dressing rather than a true skinsubstitute.) Autograft take following applicationof the synthetic membrane was excellent.
Levine notes the following advantages of thisnylon–PTFE skin:
it conforms well to irregular wound surfaces
it does not fragment and is flexible enoughto permit motion of the extremities
is semitransparent so that the wound surfacecan be observed through the dressing
Collagen-synthetic composites. In a landmarkreport in 1981, Burke and colleagues241 describedthe clinical treatment of extensive burns with anartificial skin consisting of a collagen dermis at-tached to a Silastic epidermis. The dermis was aporous sponge-like matrix of bovine type I col-lagen and glycosaminoglycans. The epidermis wasa 0.1-mm thick Silastic layer to retain moisture.When placed on a granulating bed or excised full-thickness burn wound, the dermal componentconverts to a synthesized connective tissue matrixsimilar to human dermis within 2–3 weeks.241,242
At this point the Silastic epidermis can be replacedwith a thin STSG or CEA-fibrin preparation.243,244
An alternative method was described byHansbrough and colleagues245 and consists of hu-man neonatal fibroblasts cultured in Biobrane. TheBiobrane-HF grafts adhered well to full-thicknesswounds, provided effective wound closure, andwere long lasting (up to 40 d). There was rapidfibrovascular ingrowth and minimal inflammatoryresponse associated with these cultured grafts.
Making an Informed Choice
In an editorial in Archives of Dermatology titled“New Skin for Old” and dated 1998, Phillips246
reviews developments in biological skin substitutesand addresses the following questions: (1) Whatskin substitutes are available and how does hu-man skin equivalent (HSE) compare with them?(2) How do the results of this study compare withthe standard of care for venous ulcers? (3) Whatare the risks and benefits of treatment with bio-logical skin substitutes? (4) What are the possiblemechanisms of action of these biological agents?and (5) How will these products be used in clinicalpractice? Readers are urged to read this article ifcontemplating the use of skin substitutes.
Other recommended papers exploring variousfacets of skin equivalents are by Nangia andHung,247,248 Yang,249 Grisolia et al,250 Prunieras,251
Bertolami and colleagues,252 Carver and Leigh,253
Matsuda and associates,254 Cooper and Spiel-vogel,255 and Nakazawa and coworkers.256

SRPS Vol 9, No 1, 1999
18
BIBLIOGRAPHY
1. Ratner D: Skin grafting. From here to there. Dermatol Clin16(1):75-90, 1998.
2. Hauben DJ, Baruchin A, Mahler D: On the history of thefree skin graft. Ann Plast Surg 9:242, 1982.
3. Brown JB, McDowell F: Massive repairs with thick split-skin grafts; emergency “dressing” with homografts inburns. Ann Surg 115:658, 1942.
4. Tanner JC Jr, Vandeput J, Olley JF: The mesh skin graft.Plast Reconstr Surg 34:287, 1964.
5. Rheinwald JG, Green H: Serial cultivation of strains ofhuman epidermal keratinocytes: The formation of kerati-nizing colonies from single cells. Cell 6:331, 1975.
6. Green H, Kehinde O, Thomas J: Growth of culturedepidermal cells into multiple epithelia suitable for grafting.Proc Nat Acad Sci USA 76:5665, 1979.
7. Vistnes LM: Grafting of skin. Surg Clin North Am 57:939,1977.
8. Grabb WC: Basic Techniques of Plastic Surgery (SkinGrafts). In: Grabb WC, Smith JW, Plastic Surgery, 3rd ed.Boston, Little, Brown, 1979, p 16-35.
9. Rudolph R, Klein L: Healing processes in skin grafts. SurgGynecol Obstet 136:641, 1973.
10. Robinson JB, Rohrich RJ: Wound Healing and AbnormalScars. Selected Read Plast Surg 9(1):1-35, 1999.
11. Smahel J: The healing of skin grafts. Clin Plast Surg 4:409,1977.
12. Marckmann A: Reaction of skin to autotransplantation.Studies on the microcirculation and the biochemicalcomposition in dermis (Thesis). Copenhagen, Munksgaard,1966.
13. Medawar PB: The behaviour and fate of skin autograftsand skin homografts in rabbits. J Anat 78:176, 1944.
14. Medawar PB: A second study in the behaviour and fateof skin homografts in rabbits. J Anat 79:157, 1945.
15. Medawar PB: The experimental study of skin grafts. BrMed Bull 3:79, 1945.
16. Scothorne RJ, Tough JS: Histochemical studies of humanskin autografts and homografts. Br J Plast Surg 5:161,1952.
17. Van Winkle W: The fibroblast in wound healing. SurgGynecol Obstet 124:369, 1967.
18. Grillo HC: Derivation of fibroblasts in the healing wound.Arch Surg 88:218, 1964.
19. Converse JM, Ballantyne DL Jr: Distribution of diphospho-pyridine nucleotide diaphorase in rat skin autografts andhomografts. Plast Reconstr Surg 30:415, 1962.
20. Hinshaw JR, Miller ER: Histology of healing split-thickness,full-thickness autogenous skin grafts and donor sites. ArchSurg 91:658, 1965.
21. Cramer L, Hinshaw J: Autograft rejection induced byhomografting. Plast Reconstr Surg 35:572, 1965.
22. Klein L: Reversible transformation of fibrous collagen toa soluble state in vivo. Proc Nat Acad Sci USA 62:920,1969.
23. Klein L, Vessely JC, Heiple KG: Quantification of 3-Hcollagen loss of rat allografted and isografted tendon. JBone Joint Surg 51:891, 1969.
24. Peacock EE Jr: Dynamic aspects of collagen biology. JSurg Res 7:433, 1967.
25. Dunphy JE, Udupa KN: Chemical and histochemicalsequences in the normal healing of wounds. N Engl J Med253:847, 1955.
26. Hilgert I: Changes in the hydroxyproline and hexosaminecontent of grafts after transplantation. Folia Biol (Praha)91:136, 1963.
27. Marckmann A: Autologous skin grafts in the rat. Uptakeof 3-5-S-sulfate. Proc Soc Exp Biol Med 119:557, 1965.
28. Udenfriend S: Formation of hydroxyproline in collagen.Science 152:1335, 1966.
29. Rudolph R, Klein L: Isotopic quantification of collagenturnover in skin grafts. Surg Forum 22:489, 1971.
30. Ohuchi K, Tsurufugi F: Degradation and turnover ofcollagen in the mouse skin and the effect of whole bodyx-irradiation. Biochem Biophysiol Acta 208:475, 1970.
31. Mac Neil S: What role does the extracellular matrix servein skin grafting and wound healing? Burns 20:S67, 1994.
32. Pepper FJ: Studies on the viability of mammalian skinautografts after storage at different temperatures. Br JPlast Surg 6:250, 1954.
33. Clemmesen T: The early circulation in split skin grafts.Acta Chir Scand 124:11, 1962.
34. Clemmesen T: Experimental studies on the healing of freeskin autografts. Danish Med Bull 14, Suppl 11, 1967.
35. Converse JM, Uhlschmid GK, Ballantyne DL Jr: “Plasmaticcirculation” in skin grafts. Plast Reconstr Surg 43:495, 1969.
36. Converse JM et al: Inosculation of vessels of skin graft andhost bed: A fortuitous encounter. Br J Plast Surg 28:274,1975.
37. Peer LA, Walker JC: The behaviour of autogenous humantissue grafts, I. Plast Reconstr Surg 7:6, 1951.
38. Peer LA: Transplantation of Tissues, Vol 2. Baltimore,Williams & Wilkins, 1955.
39. Birch J, Branemark PI: The vascularization of a free full-thickness skin graft. I. A vital microscopic study. ScandJ Plast Reconstr Surg 3:1, 1969.
40. Birch J, Branemark PI, Lundskog J: The vascularization ofa free full-thickness skin graft. II. A microangiographicstudy. Scand J Plast Reconstr Surg 3:11, 1969.
41. Birch J, Branemark PI, Nilsson K: The vascularization of afree full-thickness skin graft. III. An infrared thermo-graphic study. Scand J Plast Reconstr Surg 3:18, 1969.
42. Psillakis JM et al: Water and electrolyte changes inautogenous skin grafts. Discussion of the so-called “plas-matic circulation.” Plast Reconstr Surg 43:500, 1969.43. Haller JA, Billingham RE: Studies of the origin of thevasculature in free skin grafts. Ann Surg 166:896, 1967.
44. Converse JM, Rapaport FT: The vascularization of skinautografts and homografts; an experimental study in man.Ann Surg 143:306, 1956.
45. Converse JM et al: A study of viable and non-viable skingrafts transplanted to the chorio-allantoic membrane ofthe chick embryo. Transplant Bull 5:108, 1958.
46. Converse JM, Filler M, Ballantyne DL: Vascularization ofsplit-thickness skin autografts in the rat. Transplantation3:22, 1965.
47. Zarem HA, Zweifach BW, McGehee JM: Developmentof microcirculation in full thickness autogenous skin graftsin mice. Am J Physiol 212:1081, 1967.
48. Ljungvist A, Almgard IE: The vascular reaction in the freeskin allo- and autografts. Acta Path Microbiol Scand68:553, 1966.
49. Wolff K, Schellander FG: Enzyme-histochemical studieson the healing process of split skin grafts. J Invest Dermatol45:38, 1965.
50. Tsukada S: Transfer of free skin grafts with a preservedsubcutaneous vascular network. Ann Plast Surg 4:500,1980.

SRPS Vol 9, No 1, 1999
19
51. Ericksson G et al: Electron microscopic studies on theepidermis in human split-skin autografts. Scand J PlastReconstr Surg 2:83, 1968.
52. Zoltan J: Transplantationslehre. In: Gohrbandt-Gabka-Berndorfer (eds), Handbuch der Plastischen Chirurgie,Band 1, Lieferung 3, Beitrag 12. Berlin, Walter de Gruyter,1965, p 1-244.
53. Burleson R, Eiseman B: Nature of the bond betweenpartial-thickness skin and wound granulations. Am Surg177:181, 1973.
54. Tavis MJ et al: Graft adherence to de-epithelializedsurfaces: A comparative study. Ann Surg 184:594, 1976.
55. Stuart JD, Morgan RF, Kenney JG: Single-donor fibrin gluefor hand burns. Ann Plast Surg 24:524, 1990.
56. Saltz R et al: Experimental and clinical applications offibrin glue. Plast Reconstr Surg 88:1005, 1991.
57. Cederholm-Williams SA: Benefits of adjuvant fibrin gluein skin grafting. Med J Aust 161(9):575, 7 Nov 1994.
58. Dean M, Nicholls M, Wedderburn C: Benefits of adjuvantfibrin glue in skin grafting. Med J Aust 160(8):526, 18 Apr1994.
59. Achauer BM, Miller SR, Lee TE: The hemostatic effect offibrin glue on graft donor sites. J Burn Care Rehabil 15:24,1994.
60. Himel HN et al: A new device for securing meshed split-thickness skin grafts. Scand J Plast Reconstr Hand Surg28:299, 1994.
61. Himel HN et al: Biomechanical performance of a dispos-able graft stapler with absorbable skin tacs. Burns 20:244,1994.
62. Sheridan RL, Petras L, Basha G, Tompkins RG: Clinicalexperience with the use of biodegradable tacks in pediat-ric patients with burns. J Burn Care Rehabil 16:143, 1995.
63. Best T, Lobay G, Moysa G, Tredget E: A prospectiverandomized trial of absorbable staple fixation of skingrafts for burn wound coverage. J Trauma 38:915, 1995.
64. Netscher DT, Marchi M, Wigoda P: A method foroptimizing skin graft healing and outcome of wounds ofthe penile shaft and scrotum. Ann Plast Surg 31:447, 1993.
65. Saltz R, Bowles BJ: Reston: an alternate method of skingraft fixation (letter). Plast Reconstr Surg 99:601, 1997.
66. Caldwell RK, Giles WC, Davis PT: Use of foam bolsters forsecuring facial skin grafts. Ear Nose Throat J 77(6):490,1998.
67. Balakrishnan C: Dressing for skin grafts of the penis(letter). Plast Reconstr Surg 95:208, 1995.
68. Johnson PA, Fleming K, Avery CME: Latex foam andstaple fixation of skin grafts. Br J Oral Maxillofac Surg36:141, 1998.
69. Wolf Y, Kalish E, Badani E, et al: Rubber foam and staples:Do they secure skin grafts? A model analysis and proposalof pressure enhancement techniques. Ann Plast surg40:149, 1998.
70. Smoot EC: A rapid method for splinting skin grafts andsecuring wound dressings (letter). Plast Reconstr Surg100(6):1622, 1997.
71. Amir A, Sagi A, Fliss DM, Rosenberg L: A simple, rapid,reproducible tie-over dressing. Plast Reconstr Surg98(6):1092, 1996.
72. Cheng LC, Lim TC, Tan WTL: A simple tie-over dressing(letter). Plast Reconstr Surg 101(1):246, 1998.
73. Vloemans AFPM, Kreis RW: Fixation of skin grafts with anew silicone rubber dressing (Mepitel). Scand J PlastReconstr Hand Surg 28:75, 1994.
74. Sawada Y, Yotsuyanagi T, Ara M, Sone K: Experiencesusing silicone gel tie-over dressings following skin graft-ing. Burns 16(5):353, 1990.
75. Renz BM, Stout M, Sherman R: Rubberband stents forskin grafts: How I do it. Am Surg 60:707, 1994.
76. Ren J et al: Transparent gasbag tie-over for persistentpressure and inspection in free skin grafting. Plast ReconstrSurg 95:396, 1995.
77. Ward RS et al: Uses of Coban self-adherent wrap inmanagement of postburn hand grafts: Case reports. JBurn Care Rehabil 15:364, 1994.
78. Grabski WJ, Giandoni MB, Anderson LL: Surgical pearl:Hydrocolloid dressings for full-thickness skin grafts. J AmAcad Dermatol 32:273, 1995.
79. Watson SB, Miller JG: Optimizing skin graft take inchildren’s hand burns—the use of Silastic foam dressings.Burns 19:519, 1993.
80. Balakrishnan C: Simple method of applying pressure toskin grafts of neck with foam dressing and staples. J BurnCare Rehabil 15:432, 1994.
81. Wells MD, Kirn DS: A new method of skin-graft stabiliza-tion: the Reston technique. Ann Plast Surg 34:554, 1995.
82. Schneider AM, Morykwas MJ, Argenta LC: A new andreliable method of securing skin grafts to the difficultrecipient bed. Plast Reconstr Surg 102(4):1195, 1998.
83. McGregor IA, Conway H: Development of lymph flowfrom autografts and homografts of skin. Transplant Bull3:46, 1956.
84. Gloor M, Ludwig G: Revascularization of free full-thicknessskin autografts. Arch Dermatol Forsch 246:211, 1973.
85. Corps BVM: The effect of graft thickness, donor site andgraft bed on graft shrinkage in the hooded rat. Br J PlastSurg 22:125, 1969.
86. Padgett EC: Calibrated intermediate skin grafts. PlastReconstr Surg 39:195, 1967.
87. Rudolph R: The effect of skin graft preparation on woundcontraction. Surg Gynecol Obstet 142:49, 1976.
88. Bertolami C, Donoff RB: The effect of full-thickness skingrafts on the actomyosin content of contracting wounds.J Oral Surg 37:471, 1979.
89. Bertolami CN, Donoff RB: The effect of skin grafting uponprolyl hydroxylase and hyaluronidase activities in mam-malian wound repair. J Surg Res 27:359, 1979.
90. Brown D, Garner W, Young VL: Skin grafting: Dermalcomponents in inhibition of wound contraction. SouthMed J 83:789, 1990.
91. Rudolph R et al: Control of contractile fibroblasts by skingrafts. Surg Forum 28:524, 1977.
92. Rudolph R: Inhibition of myofibroblasts by skin grafts.Plast Reconstr Surg 63:473, 1979.
93. Montandon D et al: The contractile fibroblast: Its relevancein plastic surgery. Plast Reconstr Surg 52:286, 1973.
94. Oliver RF, Grant RA, Kent CM: The fate of cutaneouslyand subcutaneously implanted trypsin purified dermalcollagen in the pig. Br J Exp Pathol 53:540, 1972.
95. Oliver RF et al: Incorporation of stored cell-free dermalcollagen allografts into skin wounds: A short term study.Br J Plast Surg 30:88, 1977.
96. Waris T et al: Regeneration of cold, warmth and heat-painsensibility in human skin grafts. Br J Plast Surg 42:576,1989.
97. Haro JJ, Del Valle ME, Calzada B, et al: Human glabrousskin autografts partially reinnervated without sensorycorpuscles. An immunohistochemical study. Scand J PlastReconstr Hand Surg 28:25, 1994.

SRPS Vol 9, No 1, 1999
20
98. Stella M, Calcagni M, Teich-Alasia S, et al: Sensoryendings in skin grafts and scars after extensive burns.Burns 20(6):491, 1994.
99. Ponten B: Grafted skin. Observations on innervation andother qualities. Acta Chir Scand Suppl 257, 1960.
100. Adeymo O, Wyburn G: Innervation of skin grafts. Trans-plantation 4:152, 1957.
101. Fitzgerald MJT, Martin F, Paletta FX: Innervation of skingrafts. Surg Gynecol Obstet 124:808, 1967.
102. Weis-Becker C, Fruhstorfer H, Friederich H-C, Winter H:Reinnervation of split skin grafts in humans: comparisonof two different methods of operation. Scand J PlastReconstr Hand Surg 32:157, 1998.
103. Conway H, Sedar J: Report of the loss of pigment in fullthickness autoplastic skin grafts in the mouse. PlastReconstr Surg 18:30, 1956.
104. Mir y Mir L: The problem of pigmentation in the cutane-ous graft. Br J Plast Surg 14:303, 1961.
105. Taki T et al: Surgical treatment of skin depigmentationcaused by burn injuries. J Dermatol Surg Oncol 11:1218,1985.
106. Jarrett A, Szabo G: The pathologic varieties of vitiligo andtheir response to treatment with mladinin. Br J Dermatol68:313, 1956.
107. Falabella R: Repigmentation of leukoderma by autolo-gous epidermal grafting. J Dermatol Surg Oncol 10:136,1984.
108. Hatchome N, Kato T, Tagami H: Therapeutic success ofepidermal grafting in generalized vitiligo is limited by theKoebner phenomenon. J Am Acad Dermatol 22:87,1990.
109. Kahn AM, Cohen MJ: Vitiligo: treatment by dermabra-sion and epithelial sheet grafting—a preliminary report. JAm Acad Dermatol 28:773, 1993.
110. Behl PN: Vitiligo: treatment by dermabrasion andepithelial sheet grafting (letter). J Am Acad Dermatol30:1044, 1994.
111. Achauer BM, Le Y, Vander Kam VM: Treatment of vitiligowith melanocytic grafting. Ann Plast Surg 33:644, 1994.
112. Olsson MJ, Juhlin L: Epidermal sheet grafts forrepigmentation of vitiligo and piebaldism, with a reviewof surgical techniques. Acta Derm Venereol (Stockh)77:463, 1997.
113. Yang JS, Kye YC: Treatment of vitiligo with autologousepidermal grafting by means of pulsed erbium:YAG laser.J Am Acad Dermatol 38(2 Pt 1):280, 1998.
114. Kahn AM, Cohen MJ: Repigmentation in vitiligo patients.Melanocyte transfer via ultra-thin grafts. Dermatol Surg24:365, 1998.
115. Kaufmann R, Greiner D, Kippenberger S, Bernd A: Graftingof in vitro cultured melanocytes onto laser-ablated lesionsin vitiligo. Acta Derm Venereol (Stockh) 78:136, 1998.
116. Hosokawa K, Hata Y, Yano K, et al: Treatment of tattooswith pure epidermal sheet grafting. Ann Plast Surg 24:53,1990.
117. Webster GV: Annual Report to the American Society ofPlastic and Reconstructive Surgeons. Hollywood, Florida,October 1954.
118. Rees TD, Casson PR: The indications for cutaneousdermal overgrafting. Plast Reconstr Surg 38:522, 1966.
119. Wheeland RG: The technique and current status of pinchgrafting. J Dermatol Surg Oncol 13:873, 1987.
120. Smahel J, Ganzoni N: Relay transplantation: A newmethod of expanding a free skin graft. Br J Plast Surg28:49, 1975.
121. Knight SL, Moorghen M: Configurational changes withinthe dermis of meshed split skin grafts: a histologicalstudy. Br J Plast Surg 40:420, 1987.
122. Davison PM, Batchelor AG, Lewis-Smith PA: The prop-erties and uses of non-expanded machine-meshed skingrafts. Br J Plast Surg 39:462, 1986.
123. Glogau RG, Stegman SJ, Tromovitch TA: Refinements insplit-thickness skin grafting technique. J Dermatol SurgOncol 13:853, 1987.
124. Richard R, Miller SF, Steinlage R, Finley RK Jr: Acomparison of the Tanner and Bioplasty skin meshersystems for maximal skin graft expansion. J Burn CareRehabil 14:690, 1993.
125. Kreis RW, Mackie DP, Hermans RK, Vloemans AR:Expansion techniques for skin grafts: comparison be-tween mesh and Meek island (sandwich-) grafts. Burns20:S39, 1994.
126. Zhang ML et al: Microskin grafting. II. Clinical report.Burns 12:544, 1986.
127. Zhang ML et al: Microskin grafting in the treatment ofextensive burns: A preliminary report. J Trauma 28:804,1988.
128. Lai C-S et al: An easy way to prepare microskin grafts.Burns 20:151, 1994.
129. Lin T-W: The algebraic view-point in microskin graftingin burned patients. Burns 20:347, 1994.
130. Lin T-W, Horng S-Y: A new method of microskinmincing. Burns 20:526, 1994.
131. Vandeput J, Tanner J: Easy way to prepare microskingrafts. Burns 20:476, 1994.
132. Wang LN, Hsu JH, Lu KH: Experimental studies oncombined use of homografts and autografts. Chin MedJ 4:221, 1973.
133. Yang CC et al: The intermingled transplantation of auto-and homografts in severe burns. Burns 6:141, 1980.
134. Hatchome N, Kato T, Tagami H: Therapeutic success ofepidermal grafting in generalized vitiligo is limited by theKoebner phenomenon. J Am Acad Dermatol 22:87, 1990.
135. Christiansen J, Ek L, Tegner E: Pinch grafting of leg ulcers.A retrospective study of 412 treated ulcers in 146patients. Acta Derm Venereol (Stockh) 77:471, 1997.
136. Kirsner RS, Mata SM, Falanga V, Kerdel FA: Split-thickness skin grafting of leg ulcers. The University ofMiami Department of Dermatology’s experience (1990-1993). Dermatol Surg 21:701, 1995.
137. Yeh FL, Yu GS, Fang CH, et al: Comparison of scarcontracture with the use of microskin and Chinese-typeintermingled skin grafts on rats. J Burn Care Rehabil11:221, 1990.
138. Flowers R: Unexpected postoperative problems in skingrafting. Surg Clin North Am 50:439, 1970.
139. Teh BT: Why do skin grafts fail? Plast Reconstr Surg63:323, 1979.
140. Hill TG: Enhancing the survival of full-thickness grafts. JDermatol Surg Oncol 10:639, 1984.
141. Wolfort S, Rohrich RJ, Handren J, May JW Jr: The effectof epinephrine in local anesthesia on the survival of full-and split-thickness skin grafts: an experimental study.Plast Reconstr Surg 86(3):535, 1990.
142. Fazio MJ, Zitelli JA: Full-thickness skin grafts. Clinicalobservations on the impact of using epinephrine in localanesthesia of the donor site. Arch Dermatol 131:691,1995.
143. Robson MC, Krizek TJ: Predicting skin graft survival. JTrauma 13:213, 1973.

SRPS Vol 9, No 1, 1999
21
144. Perry AW et al: Skin graft survival—The bacterial answer.Ann Plast Surg 22:479, 1989.
145. Allen HE et al: Skin dressings in the treatment ofcontaminated wounds. Am J Surg 126:45, 1973.
146. Szabo SE, Toomey JM, Linn BS: Does skin have antimi-crobial properties? An in-vitro experiment and literaturereview. Am Surg 44:55, 1978.
147. Feldman DL: Which dressing for split-thickness skin graftdonor sites? Ann Plast Surg 27:288, 1991.
148. Gallico GG III: Biologic skin substitutes. Clin Plast Surg17:519, 1990.
149. Hoekstra MJ, Kreis RW, du Pont JS: History of the EuroSkin Bank: the innovation of preservation technologies.Burns 20:S43, 1994.
150. Basile ARD: A comparative study of glycerinized andlyophilized porcine skin dressings for third degree burns.Plast Reconstr Surg 69:969, 1982.
151. Backere ACJ: Euro Skin Bank: large scale skin-bankingin Europe based on glycerol-preservation of donor skin.Burns 20:S4, 1994.
152. Hettich R, Ghofrani A, Hafemann B: The immunogenic-ity of glycerol-preserved donor skin. Burns 20:S71, 1994.
153. Baare J, Buitenwerf J, Hoekstra MJ, du Pont JS: Virucidaleffect of glycerol as used in donor skin preservation.Burns 20:S77, 1994.
154. Pirnay JP, Vandenvelde C, Duinslaeger L, et al: HIVtransmission by transplantation of allograft skin: a re-view of the literature. Burns 23:1, 1997.
155. Simonds RJ: HIV transmission by organ and tissuetransplantation. AIDS 7:35, 1993.
156. Asselmeier MA, Caspari RB, Bottenfield S: A review ofallograft processing and sterilization techniques andtheir role in transmission of the human immunodefi-ciency virus. Am J Sports Med 21:170, 1993.
157. Buck BE, Resnick I, Shah SM, Malinin TI: Humanimmunodeficiency virus cultured from bone: implica-tions for transplantation. Clin Orthop 251:249, 1990.
158. Conway B, Tomford W, Mankin HJ, et al: Radiosensitiv-ity of HIV-1: potential application to sterilisation of boneallografts. AIDS 5:608, 1991.
159. Brans TA, Hoekstra MJ, Vloemans AFPM, Kreis RW:Long-term results of treatment of scalds in children withglycerol-preserved allografts. Burns 20:S10, 1994.
160. Peeters R, De Caluwe D, Neetens C, Hubens A: Use ofglycerolized cadaver skin for the treatment of scalds inchildren. Burns 20:S32, 1994.
161. Hussmann J et al: Use of glycerolized human allograftsas temporary (and permanent) cover in adults andchildren. Burns 20:S61, 1994.
162. Schiozer WA et al: Composite grafts of autogeniccultured epidermis and glycerol-preserved allogeneicdermis for definitive coverage of full thickness burnwounds: case reports. Burns 20:503, 1994.
163. McKay I et al: Reconstruction of human skin fromglycerol-preserved allodermis and cultured keratinocytesheets. Burns 20:S19, 1994.
164. Phipps AR, Clarke JA: The use of intermingled autograftand parental allograft skin in the treatment of majorburns in children. Br J Plast Surg 44:608, 1991.
165. Dohi K et al: Studies of alloantibody effect on skin graftsurvival in mice. Hiroshima J Med Sci 33:271, 1984.
166. Dohi K et al: Studies of immunosuppressive effect ofanti-recipient alloantiserum. I. Effect on skin graftsurvival in mice. Hiroshima J Med Sci 33:507, 1984.
167. Steines NA, Gug K, Davies DAL: Passive enhancementof mouse skin allografts; specificity of the antiserum formajor histocompatibility complex antigens. Transplanta-tion 18:192, 1974.
168. McCullough CS, Sugarbaker PH, Matthews W: Effects ofpassive enhancement on graft and host. Transplantation37:91, 1984.
169. Towpik E et al: Cyclosporine and experimental skinallografts: Long-term survival in rats treated with lowmaintenance doses. Plast Reconstr Surg 77:268, 1986.
170. Towpik E: Meshed allograft skin and cyclosporin immu-nosuppression (letter). Br J Plast Surg 42:618, 1989.
171. Kolenik SA III, Leffell DJ: The use of cryopreservedhuman skin allografts in wound healing following Mohssurgery. Dermatol Surg 21:615, 1995.
172. Csönge L, Pellet S, Szenes A, István J: Antibiotics in thepreservation of allograft and xenograft skin. Burns21(2):102, 1995.
173. Robson MC, Krizek TJ: The effect of human amnioticmembranes on the bacterial population of infected ratburns. Ann Surg 177:144, 1973.
174. Rao TV, Chandrasekharam V: Use of dry human andbovine amnion as a biological dressing. Arch Surg116:891, 1981.
175. Waikakul S et al: Application of freeze-dried amnioticmembrane: A control trial at the donor site of split-thickness skin grafting. Bull Hosp Jt Dis Orthop Inst50:27, 1990.
176. (Anonymous): Anyone for amnion? (editorial). Lancet1:719, Mar 31, 1984.
177. Feldman DL, Karpinski RHS: A prospective trial compar-ing Biobrane, Duoderm, and xeroform for skin graftdonor sites. Surg Gynecol Obstet 173:1, 1991.
178. Morris WT, Lamb AM: Painless split skin donor sites: Acontrolled double-blind trial of Op-Site, scarlet red, andbupivacaine. Aust NZ J Surg 60:617, 1990.
179. Brady SC, Snelling CFT, Chow G: Comparison of donorsite dressings. Ann Plast Surg 5:238, 1980.
180. Barnett A et al: Comparison of synthetic adhesivemoisture vapor permeable and fine mesh gauze dress-ings for split-thickness skin graft donor sites. Am J Surg145:379, 1983.
181. Zapata-Sirvent R et al: Comparison of Biobrane andScarlet Red dressings for treatment of donor site wounds.Arch Surg 120:743, 1985.
182. Tavis MJ et al: A new composite skin prosthesis. Burns7:123, 1981.
183. Poulsen TD, Freund KG, Arendrup K, et al: Polyurethanefilm (Opsite*) vs. impregnated gauze (Jelonet*) in thetreatment of outpatient burns: a prospective, random-ized study. Burns 17:59, 1991.
184. Lawrence JE, Blake GB: A comparison of calciumalginate and scarlet red dressings in the healing of splitthickness skin graft donor sites. Br J Plast Surg 44:247,1991.
185. Porter JM: A comparative investigation of re-epithelialisation of split skin graft donor areas afterapplication of hydrocolloid and alginate dressings. Br JPlast Surg 44:333, 1991.
186. Nemeth AJ et al: Faster healing and less pain in skinbiopsy sites treated with an occlusive dressing. ArchDermatol 127:1679, 1991.
187. Sawada Y, Yotsuyanagi T, Sone K: A silicone gel sheetdressing containing an antimicrobial agent for split thick-ness donor site wounds. Br J Plast Surg 43:88, 1990.

SRPS Vol 9, No 1, 1999
22
188. Owen TD, Dye D: The value of topical lignocaine gel inpain relief on skin graft donor sites. Br J Plast Surg 43:480,1990.
189. Alvi R, Jones S, Burrows D, et al: The safety of topicalanaesthetic and analgesic agents in a gel when used toprovide pain relief at split skin donor sites. Burns 24:54,1998.
190. Pruitt BA Jr, Levine NS: Characteristics and uses ofbiologic dressings and skin substitutes. Arch Surg 119:312,1984.
191. Hull BE, Finley RK, Miller SF: Coverage of full-thicknessburns with bilayered skin equivalents: A preliminaryclinical trial. Surgery 107:496, 1990.
192. Harriger MD et al: Pigmentation and microanatomy ofskin regenerated from composite grafts of cultured cellsand biopolymers applied to full-thickness burn wounds.Transplantation 59:702, 1995.
193. Maruguchi T et al: A new skin equivalent: keratinocytesproliferated and differentiated on collagen sponge con-taining fibroblasts. Plast Reconstr Surg 93:537, 1994.
194. Beumer GJ, van Blitterswijk CA, Ponec M: Biocompat-ibility of a biodegradable matrix used as a skin substitute:an in vivo evaluation. J Biomed Mat Res 28:545, 1994.
195. Ghosh MM, Boyce S, Layton C, et al: A comparison ofmethodologies for the preparation of human epidermal-dermal composites. Ann Plast Surg 39:390, 1997.
196. O’Connor NE et al: Grafting of burns with culturedepithelium prepared from autologous epidermal cells.Lancet 1:75, 1981.
197. Phillips TJ, Kehinde O, Green H, Gilchrest BA: Treatmentof skin ulcers with cultured epidermal allografts. J AmAcad Dermatol 21:191, 1989.
198. Teepe RGC, Burger A, Ponec M: Immunohistochemicalstudies on regeneration in cultured epidermal autograftsused to treat full-thickness burn wounds. Clin ExpDermatol 19:16, 1994.
199. Phillips TJ et al: Cultured epidermal autografts andallografts: A study of differentiation and allograft sur-vival. J Am Acad Dermatol 23:189, 1990.
200. Carver N, Leigh IM: Keratinocyte grafts and skin equiva-lents. Int J Dermatol 30:540, 1991.
201. Brain A et al: Survival of cultured allogeneic keratinocytestransplanted to deep dermal bed assessed with probesspecific for Y chromosome. Br Med J 298:1917, 1989.
202. Burt AN et al: Survival of cultured allografts in patientswith burns assessed with probes specific for Y chromo-some. Br Med J 298:915, 1989.
203. Oliver AM et al: The differentiation and proliferation ofnewly formed epidermis on wounds treated with cul-tured epithelial allografts. Br J Dermatol 125:147, 1991.
204. Fabre JW: Epidermal allografts. Immunol Lett 29:161,1991.
205. Tomson AM et al: Improved in vitro generation of epithe-lial grafts with oral mucosa. Transplantation 58:1282, 1994.
206. Donati L et al: Clinical experiences with keratinocytegrafts. Burns (Suppl 1):519, 1992.
207. Kumagai N et al: Clinical applications of autologouscultured epithelia for the treatment of burn wounds andburn scars. Plast Reconstr Surg 82:99, 1988.
208. Green H: Cultured cells for the treatment of disease. SciAm 265:96, 1991.
209. Herndon DN, Rutan RL: Comparison of cultured epider-mal autograft and massive excision with serial autograftingplus homograft overlay. J Burn Care Rehabil 13:154,1992.
210. Desai MH et al: Lack of long-term durability of culturedkeratinocyte burn-wound coverage: A case report. JBurn Care Rehabil 12:540, 1991.
211. Blight A et al: The treatment of donor sites with culturedepithelial grafts. Br J Plast Surg 44:12, 1991.
212. Blight A et al: Treatment of full skin thickness burn injuryusing cultured epithelial grafts. Burns 17:495, 1991.
213. Munster AM, Weiner SH, Spence RJ: Cultured epider-mis for the coverage of massive burn wounds. A singlecenter experience. Ann Surg 211:676, 1990.
214. Petersen MJ, Lessane B, Woodley DT: Characterizationof cellular elements in healed cultured keratinocyteautografts used to cover burn wounds. Arch Dermatol126:175, 1990.
215. Clugston PA et al: Cultured epithelial autografts: Threeyears of clinical experience with eighteen patients. JBurn Care Rehabil 12:533, 1991.
216. Ronfard V et al: Use of human keratinocytes cultured onfibrin glue in the treatment of burn wounds. Burns17:181, 1991.
217. Nanchahal J, Davies D: Cultured composite skin graftsfor burns. Br Med J 301:1342, 1990.
218. Krupp S et al: Mid-term results with cultured epidermalautografts, allogenic skin transplants and cyclosporin Amedication. Burns 20:15, 1994.
219. Hickerson WL, Compton C, Fletchall S, Smith LR: Culturedepidermal autografts and allodermis combination for per-manent burn wound coverage. Burns 20:S52, 1994.
220. Latarjet J et al: The grafting of burns with culturedepidermis as autografts in man. Scand J Plast ReconstrSurg 21:241, 1987.
221. Thivolet J et al: Long-term survival and immunologicaltolerance of human epidermal allografts produced inculture. Transplantation 42:274, 1986.
222. Still JM Jr, Orlet HK, Law EJ: Use of cultured epidermalautografts in the treatment of large burns. Burns 20:539,1994.
223. Sheridan RL, Tompkins RG: Cultured autologous epithe-lium in patients with burns of ninety percent or more ofthe body surface. J Trauma 38:48, 1995.
224. Skolnick AA: Undifferentiated cell transplant techniquesappear effective in treating leg ulcers, vitiligo. JAMA266:1331, 1991.
225. Carter DM et al: Treatment of junctional epidermolysisbullosa with epidermal autografts. J Am Acad Dermatol172:246, 1987.
226. Dean SJ, Nieber S, Hickerson WL: The use of culturedepithelial autograft in a patient with idiopathic pyo-derma gangrenosum. Ann Plast Surg 26:194, 1991.
227. McGrath J, Black M: Split skin grafting and bullouspemphigoid. Clin Exp Dermatol 16:72, 1991.
228. Gallico GG, O’Connor NE, Compton CC: Culturedepithelial autografts for giant congenital nevi. PlastReconstr Surg 84:1, 1989.
229. Premachandra DJ et al: Treatment of postoperativeotorrhea by grafting of mastoid cavities with culturedautologous epidermal cells. Lancet 1:365, 1990.
230. Blight A, Fatah MF, Datubo-Brown DD, et al: Thetreatment of donor sites with cultured epithelial grafts.Br J Plast Surg 44:12, 1991.
231. Beele H et al: Repeated cultured epidermal allografts inthe treatment of chronic leg ulcers of various origins.Dermatologica 183:31, 1991.
232. Mol MAE et al: Grafting of venous leg ulcers. Anintraindividual comparison between cultured skin equiva-

SRPS Vol 9, No 1, 1999
23
lents and full-thickness skin punch grafts. J Am AcadDermatol 24:77, 1991.
233. Brysk MM et al: Grafting of leg ulcers with undifferenti-ated keratinocytes. J Am Acad Dermatol 25:238, 1991.
234. Phillips TJ, Bigby M, Bercovitch L: Cultured allografts asan adjunct to the medical treatment of problematic legulcers. Arch Dermatol 127:799, 1991.
235. De Luca M et al: Treatment of leg ulcers with cryopre-served allogeneic cultured epithelium. A multicenterstudy. Arch Dermatol 128:633, 1992.
236. Roenigk RK et al: Dermatologic Surgery: Principles andPractice. New York, BC Decker, 1989.
237. Brown AS, Barot LR: Biologic dressings and skin substi-tutes. Clin Plast Surg 13:69, 1986.
238. Kickhofen B, Wokalek H, Scheel D, Ruh H: Chemicaland physical properties of a hydrogel wound dressing.Biomaterials 7:67, 1986.
239. Eloy R et al: Physical and biological properties of a newsynthetic amino acid copolymer used as a wound dress-ing. J Biomed Mater Res 26:695, 1992.
240. Levine NS, Peterson HD, Mason AD Jr: Use of syntheticdressing on denuded wounds in burn patients. Ann ResProg Rep, Ft Sam Houston, TX, US Army Inst Surg Res,Brooke Army Med Ctr, June 1975, pp 343-363.
241. Burke JF et al: Successful use of a physiologicallyacceptable artificial skin in the treatment of extensiveburn injury. Ann Surg 194:413, 1981.
242. Suzuki S et al: Further applications of “bilayer artificialskin.” Br J Plast Surg 48:222, 1995.
243. Suzuki S et al: Clinical evaluation of a new bilayer“artificial skin” composed of collagen sponge and sili-cone layer. Br J Plast Surg 43:47, 1990.
244. Suzuki S et al: Experimental study of a newly developedbilayer artificial skin. Biomaterials 11:356, 1990.
245. Hansbrough JF, Morgan J, Greenleaf G, Underwood J:Development of a temporary living skin replacement com-posed of human neonatal fibroblasts cultured in Biobrane,a synthetic dressing material. Surgery 115:633, 1994.
246. Phillips TJ: New skin for old. Developments in biologicalskin substitutes (editorial). Arch Dermatol 134:344, 1998.
247. Nangia A, Hung CT: Preclinical evaluation of skin substi-tutes. Burns 16(5):358, 1990.
248. Nangia A, Hung CT: Laboratory evaluation of a newhydrogel-type skin substitute. Burns 16(5):368, 1990.
249. Yang J-Y: Clinical application of collagen sheet, YCWM,as a burn wound dressing. Burns 16(6):457, 1990.
250. Grisolia GA, Pelli P, Pinzauti E, et al: Skin substitutes in thetreatment of deep partial skin thickness burns in children:clinical experience and long-term results. Burns 17(1):52,1991.
251. Prunieras M: To reconstitute skin: theme and variations.Matrix 11:302, 1991.
252. Bertolami CN, Shetty V, Milavec JE, et al: Preparation andevaluation of a nonproprietary bilayer skin substitute.Plast Reconstr Surg 87(6):1089, 1991.
253. Carver N, Leigh IM: Synthetic dressings. Int J Dermatol31(1):10, 1992.
254. Matsuda K, Suzuki S, Isshiki N, Ikada Y: Re-freeze driedbilayer artificial skin. Biomaterials 14(13):1030, 1993.
255. Cooper ML, Spielvogel RL: Artificial skin for woundhealing. Clin Dermatol 12:183, 1994.
256. Nakazawa K, Nakazawa H, Sahuc F, et al: Pigmentedhuman skin equivalent: new method of reconstitution bygrafting an epithelial sheet onto a non-contractile dermalequivalent. Pigment Cell Res 10:382, 1997.

SRPS Vol 9, No 1, 1999
24
SUGGESTED READING:
Hauben DJ, Baruchin A, Mahler D: On the history of the free skin graft. Ann Plast Surg 9:242, 1982.
Stuart JD, Morgan RF, Kenney JG: Single-donor fibrin glue for hand burns. Ann Plast Surg 24:524, 1990.
Sawada Y, Yotsuyanagi T, Ara M, Sone K: Experiences using silicone gel tie-over dressings followingskin grafting. Burns 16(5):353, 1990.
Haro JJ, Del Valle ME, Calzada B, et al: Human glabrous skin autografts partially reinnervated withoutsensory corpuscles. An immunohistochemical study. Scand J Plast Reconstr Hand Surg 28:25, 1994.
Hatchome N, Kato T, Tagami H: Therapeutic success of epidermal grafting in generalized vitiligo islimited by the Koebner phenomenon. J Am Acad Dermatol 22:87, 1990.
Kirsner RS, Mata SM, Falanga V, Kerdel FA: Split-thickness skin grafting of leg ulcers. The University ofMiami Department of Dermatology’s experience (1990-1993). Dermatol Surg 21:701, 1995.
Yeh FL, Yu GS, Fang CH, et al: Comparison of scar contracture with the use of microskin and Chinese-type intermingled skin grafts on rats. J Burn Care Rehabil 11:221, 1990.
Fazio MJ, Zitelli JA: Full-thickness skin grafts. Clinical observations on the impact of using epinephrinein local anesthesia of the donor site. Arch Dermatol 131:691, 1995.
Feldman DL, Karpinski RHS: A prospective trial comparing Biobrane, Duoderm, and xeroform for skingraft donor sites. Surg Gynecol Obstet 173:1, 1991.
Ghosh MM, Boyce S, Layton C, et al: A comparison of methodologies for the preparation of humanepidermal-dermal composites. Ann Plast Surg 39:390, 1997.
Blight A, Fatah MF, Datubo-Brown DD, et al: The treatment of donor sites with cultured epithelialgrafts. Br J Plast Surg 44:12, 1991.
Beele H, Naeyaert JM, Goeteyn M, et al: Repeated cultured epidermal allografts in the treatment ofchronic leg ulcers of various origins. Dermatologica 183:31, 1991.
Phillips TJ: New skin for old. Developments in biological skin substitutes (editorial). Arch Dermatol134:344, 1998.
Nangia A, Hung CT: Preclinical evaluation of skin substitutes. Burns 16(5):358, 1990.
Bertolami CN, Shetty V, Milavec JE, et al: Preparation and evaluation of a nonproprietary bilayer skinsubstitute. Plast Reconstr Surg 87(6):1089, 1991.
Carver N, Leigh IM: Synthetic dressings. Int J Dermatol 31(1):10, 1992.
Matsuda K, Suzuki S, Isshiki N, Ikada Y: Re-freeze dried bilayer artificial skin. Biomaterials 14(13):1030,1993.
Cooper ML, Spielvogel RL: Artificial skin for wound healing. Clin Dermatol 12:183, 1994.