7 Cervical Anatomy and Function in Turtles et al 2008...7 Cervical Anatomy and Function in Turtles...
Transcript of 7 Cervical Anatomy and Function in Turtles et al 2008...7 Cervical Anatomy and Function in Turtles...
���
7 CervicalAnatomyandFunctioninTurtles
Anthony Herrel, Johan Van Damme, and Peter Aerts
Contents
7.1 Introduction......................................................................................................................... 1637.2 MaterialsandMethods....................................................................................................... 165
7.2.1 AnatomicalStudies.................................................................................................. 1657.2.2 KinematicAnalyses................................................................................................. 165
7.3 Results................................................................................................................................. 1667.3.1 Osteology................................................................................................................. 1667.3.2 CervicalJoints......................................................................................................... 1677.3.3 Musculature............................................................................................................. 169
7.3.3.1 Chelodina................................................................................................... 1697.3.3.2 Apalone...................................................................................................... 175
7.3.4 NeckMovements...................................................................................................... 1797.3.4.1 NeckRetractionandCervicalMobilityinApalone ferox......................... 1797.3.4.2 KinematicsofSnorkelinginC. longicollis................................................ 180
7.4 Discussion........................................................................................................................... 1817.4.1 VertebralStructure.................................................................................................. 1817.4.2 CervicalMusculature............................................................................................... 1817.4.3 MovementPatterns.................................................................................................. 183
Acknowledgments.......................................................................................................................... 184References...................................................................................................................................... 184
�.� introduCtion
Beingmobileisanessentialrequirementforanyanimal.Notonlydoanimalsneedtomoveabouttofindfoodorpartners,theyalsoneedtobeabletoescapepotentialpredators(Irschick&Gar-land,2001).Interestingly,somevertebrategroupsappeartohavesacrificedpartoftheirmobilityinresponse topredationpressureby thedevelopmentofarobustarmoredbody(e.g.,pangolins,glyptodonts, turtles, and soon).Althoughbodyarmor canprovideananimalwith anadequateprotectionagainstpredators,italsodramaticallyreducesitslocomotorabilityandoverallagility(Wrenetal.,1998;Zanietal.,2005).Thus,manyarmoredvertebrateshavespecializedoneatingnon-mobilefooditemslikeplants,orclumpedfoodsourcessuchasantsortermites(King,1996).However,somegroupshavedevelopedanalternativestrategyforcapturingelusivepreybydevel-opinglong,mobileappendagessuchasprojectiletongues(Debanetal,1997;Herreletal.,2000)oralongneck(Gans,1992).Forinstance,manysemi-aquaticandaquaticturtleshavedevelopedremarkablylongnecksthatareusedtocaptureelusivepreyunderwater(Pritchard,1984).
Althoughtheturtlecarapaceprovidesanexcellentdefenseagainstpredators,itisimperativethat the longneckandheadcanbeprotectedaswell.Todo so, thehead-neck systemneeds tobewithdrawnwithinthemarginsofthebonyshell.Thiscanbedoneinoneoftwoways:inthe
3339.indb 163 11/26/07 12:07:41 PM
��� BiologyofTurtles
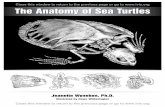
mid-sagittalplane(vertically),whichinvolvestheretractionoftheheadandneckwithinthebonyshell,orlaterally(inthehorizontalplane),wheretheneckisfoldedbetweenthedorsalandventralrimofthebonyshell.Inthiscase,theheadandneckdoremainpartiallyexposedintheoutercara-pacialchamberinfrontofthepectoralgirdle(VanDammeetal.,1995).ThisdifferenceinthemodeofneckretractioninturtleshasoftenbeenusedasanimportantcharacterforthesubdivisionoftheclassTestudinesintothesubclassesCryptodiraandPleurodira.Whereascryptodiresretracttheirhead-necksystemintheverticalplane,pleurodiresdosointhehorizontalplane(Figure7.1).
Accuratecontroloftheneckduringrapidmovementsassociatedwithescapeheadretractionandpreycaptureappearscrucialforturtles.However,theturtleneckisahighlycomplexmulti-jointedsystem,consistingofeightcervicalvertebrae,thehead,andthebodyandalargenumberofmusclesthatspananywherefromonetoovereightjoints.Thecontrolofsuchamulti-jointsystemwithalargenumberofdegreesoffreedomappearsinherentlycomplex.Althoughsomewaystofacilitatethecontrolofthesystemhavebeenidentifiedpreviously(Aertsetal.,2001),abetterunderstandingof thedetailedstructureandfunctionof themusculo-skeletalelementsof thecervicalsystemisessentialtogainbetterinsightintothecontrolofthecervicalsystem.Moreover,largedifferencesinvertebralstructure,inthemorphologyofassociatedmusculature,andinthecontrolofthecra-nio-cervicalsystemcanbeexpectedforturtlesthatretracttheirneckspredominantlyineitherthehorizontalorverticalplane.Unfortunately,previousauthorshavedescribeddifferencesinvertebralstructurebetweencryptrodiranandpleurodiranturtlespredominantlyfromataxonomicstandpoint(Vaillant,1881;Williams,1950;butseeWeisgram&Splechtna1990,1992).Thus,itremainspres-entlyunclearwhetherdifferentfunctionalcapacities(i.e.,rangesofmobility)areassociatedwitheithermorphology.Withoutthistypeofinformation,ourunderstandingofthecontrolofthecranio-cervicalsystemincryptodiranandpleurodiranturtlesmust,unfortunately,remainlimited.
Theaimof thepresent chapter is togiveadetailedmorphologicaldescriptionof thecervi-calsysteminpleurodiranandcryptodiranturtles.Indoingso,ouremphasiswillbeonthefunc-tionalconsequencesofdifferencesinmorphologyinthetwogroups.Additionally,somepreviouslyunpublisheddataon theactualkinematicsofneckmovementwillbepresented tohighlight theconsequencesofmorphologicaldifferencesinthetwogroups.Asourtyperepresentativesforcryp-todiranandpleurodiranturtleswehavechosenthegeneraChelodinaandApalone.TheAustralianpleurodiranturtlesofthegenusChelodinaarerenownedfortheirextremeelongatedneck.Becausetheneckislongerthanthecarapace,theseturtlesarealsoknownassnake-neckedturtles.Theseanimalsusetheirelongatedneckbothforquickstrikesatpreyandforsnorkeling,thusaspiratingairfromthesurfacewithoutexposingmorethanthetipofthesnout.Speciesfromthisgenuswillbeusedasatypicalrepresentativeofthepleurodirancondition.TurtlesofthegenusApalone,alsoknownasthesoft-shelledturtles,representthecryptodirancounterpartofChelodina.Theseani-malsarealsocharacterizedbyanextremelyelongatedneck,oftenlongerthanthecarapaceitself(Ernst&Barbour,1989).Soft-shellturtlestypicallyliveinshallowwaterandwillusetheirelon-gatenecktobreatheatthewatersurfacewithminimalmovement.Moreover,justlikeChelodina,
Cryptodira Pleurodira
figure�.� Schematicrepresentationofthetwomajormodesofheadretractioninturtles.Left,lateralviewonthecervicalsysteminacryptodire.Incryptodires,theheadisretractedintheverticalplane.Right,dorsalviewonthecervicalsysteminapleurodire.Heretheheadisretractedinthehorizontalplane.
3339.indb 164 11/26/07 12:07:42 PM
CervicalAnatomyandFunctioninTurtles ���
membersofthegenusApalonearevoraciouspredatorsthatwillactivelystrikeatelusivepreyunderwater(Dalrymple,1977;Ernst&Barbour,1989).
�.� materialsandmethods
7.2.1 AnAtomIcAlStudIeS
The anatomy of the cervical vertebrae, joint structures, and cervical musculature of Chelodinalongicolliswasstudiedbymeansofdissectionofapreservedspecimen(K.B.I.N.,R.G.nr.4566).Additionally,anadultC. reimani(cadaverobtainedthroughthecommercialpettrade)wasusedtostudythecervicalanatomy.OneindividualofthespeciesApalone feroxandoneA. spiniferusweredissectedtoinvestigatetheanatomyofthecervicalsysteminatypicalcryptodire.Animalswereobtainedthroughthecommercialpettrade,sacrificedbymeansofanoverdoseofNembutalandpreservedina10%aqueousformaldehydesolutionfor24hours.Next,animalswererinsedexten-sivelyandtransferredtoa70%aqueousethanolsolution.
Cervicalmorphologywasstudiedbymeansofdissections.Inthemorphologicaldescriptions,vertebraeareindicatedbycapitalCorD(cervicalanddorsalvertebrae,respectively)followedbytheirserialnumber.C1isclosesttothehead.Jointsarelabeledbythenumberoftheadjacentver-tebrae:C(n)-(n −1),withnthenumberofthemorecaudalvertebraand(n −1)thenumberofthemorecranialoneassuggestedbyHeidweiller(1991).Forexample,thejointbetweenvertebra5and6isdefinedas“jointC6-5.”
7.2.2 kInemAtIcAnAlySeS
Tostudythekinematicsofsnorkeling,twoliveadultspecimensofChelodina longicolliswereusedfortheexperiments(onefemaleof730g,18cmcarapacelengthandonemaleof520g,15cmcara-pacelength).TheanimalswereobtainedwiththehelpoftheAntwerpZooandwerehousedinaglassaqua-terrariumona12-hourlight/darkcycle.OneliveApalone feroxandoneliveA. spiniferus,obtainedthroughthecommercialpettrade,wereusedtostudythemobilityofthecervicalvertebraeintheselong-neckedcryptodires.Thewatertemperaturewaskeptat28°Cforallspecies.Twiceaweek,theturtleswerefedwithmeat,mice,andsmallinvertebrates(crickets,grasshoppers).
Snorkelingmovements(neckmovementsintheverticalplane)ofC. longicolliswererecordedbymeansofcineradiographyinlateralviewusingaPolydoros80SgeneratorequippedwithaSiemensSiregraphD40x-rayflashapparatusat66kV.Thedigitalcineradiographicrecordings(dependingonthesequence4or6framespersecond,FluorospotH)wereprintedonScopixlaserfilm(35×43cm)bymeansofanAgfalaserprinter.Duringtherecordings,theanimalswererestrainedbymeansofabody-shapedcorselet.Thiscorseletwasmountedunderthewatersurfaceonafixedframe.
Thesequenceswereprojectedframebyframeanddigitized.Digitizationofthepositionofthejointsallowedthecalculationofseveralkinematicalparameters(jointangles,elevationofthehead,headposition)inaturtle-boundframe.Thesameterminologyofjointrotationsisusedasdescribedfortheneckmovementsinthehorizontalplane,i.e.,clockwiserotationsaredefinedaspositive.
MobilityofthecervicalvertebraeinA. feroxandA. spiniferuswasstudiedbymeansofCTscanning.TheanimalwasanesthetizedbymeansofintramuscularinjectionofKetamine(150mg/kgbodymass).CTscanswererecordedusingaCT-highlightAdvantagescannerattheUniversityofAntwerpHospital.Of10different staticneckpositions, ranging fromfullyextended to fullyretracted,3-slongrecordingsresultingin1.5mmthickslicesthroughthevertebralcolumnweremadeat140kV,140mA.ScanswereprintedonScopixLT2B-100NIFx-rayfilm.
3339.indb 165 11/26/07 12:07:42 PM
��� BiologyofTurtles
�.� results
7.3.1 oSteology
Thecervicalcolumninallrecentturtlesconsistsofeightelongatedvertebrae(C1toC8)andninejoints.Themostimportantelementofthevertebraeisthevertebralcentrum,whichispositionedrightbelow the spinalcord.From thecentrum, theneuralarch risesandcovers the spinalcord(Figure7.2).Theneuralspinesarepositionedmidventrallyontheneuralroofandarereducedinmost turtles (Figure7.2).Thearticulationof thedifferentvertebraeoccursbymeansof thever-tebral centra and the zygapophyses (Figure7.2).Theprezygapophysesof thevertebra articulatewiththepostzygapophysesofthemorecraniallysituatedvertebra.Whereasthearticularfacetsoftheprezygapophysesarepositioneddorsally,thoseofthepostzygapophysesareorientedventrally.Vertebralcentracanbebiconvex(C5andC8inChelodina),biconcaveoramphicoelous(C1inbothspecies;C7 inChelodina), procoelous (C6 inChelodina) or opisthocoelous (C2,C3, andC4 inChelodina;C2toC8inApalone).
Inatransversesection, thevertebralcentrumofChelodina issaddle-shaped(Figure7.3andFigure7.4).Thevertebralcentrumiswelldevelopedonlyattheleveloftheanteriorandposteriorarticulations.Theneuralspineisreducedtoaninconspicuouslongitudinalrim.Theanteriorzyg-apophysesareclearlyseparatedfromeachother(Figure7.3andFigure7.4).Theirarticularfacetsaremainlyorientedinthemedio-dorsaldirection.TheposteriorzygapophysesarefusedtoeachotherexceptthoseofC1(Figure7.3),butthearticularfacetsremainseparatedandfacemainlyven-trallyandslightlylaterally(Figure7.3andFigure7.4).Theelevationoftheposteriorzygapophysisgraduallyincreasesfromdistaltoproximal(Figure7.3andFigure7.4).Thetransverseprocessesarestronglydeveloped,especiallythoseonthemoreproximalvertebrae(Figure7.3andFigure7.4).
Thecervicalvertebrae inApalone are relativelygracileandelongated (Figure7.5andFig-ure7.6).Theybearwell-developedandwell-separatedpre-andpost-zygapophyses(Figure7.5and
A
Posterior zygapophysis
Caudal
Centrum
Ventral
Transverse process
BPosterior zygapophysisArticular facets
posterior zygapophysisAnterior zygapophysis
Neural canal
Transverse process
Posterior articular facetof the centrum
C Posterior zygapophysis
Anterior zygapophysis
Neural canal
Transverse process
Anterior articular facetof the centrum
Cranial
Anterior zygapophysisNeural arch
Dorsal
figure�.� Chelodina longicollis.Terminologyofthemostimportantstructuralelementsofacervicalverte-bra(C7).(A)Lateralview,(B)posteriorview,and(C)anteriorview(terminologyafterRomer&Parsons,1977).
3339.indb 166 11/26/07 12:07:44 PM
CervicalAnatomyandFunctioninTurtles ���
Figure7.6).Theorientationofthezygapophysesisvariablethroughouttheneck.Becauseoftheabsence of the iliocostalis and logissimus groups (discussed later), the transverse processes areonlypoorlydeveloped(Figure7.5andFigure7.6).Becauseof thestrongdivergenceof thepost-zygapophysesandtheabsenceofawell-developedneuralspine,ahiatusintervertebralis(Bojanus,1821)iscreated.InApalone,thishiatusiscoveredbyathickmembrane(Ogushi,1913).
7.3.2 cervIcAlJoIntS
Joint S(kull)-C1 (occipito-cervical joint). This procoelous ball-and-socket type joint allows theskulltomoveindependentlyfromtheneck.Theoccipitalisspherical.Theridgesofthecongru-entcotyleonC1arewelldeveloped.C1lacksanteriorzygapophyses.Theconnectionbetweenthe
Lateral view
Dorsal view
Frontal view
Caudal view
C1 C2 C3 C4
figure�.� Lateral,dorsal, frontal,andcaudalviewsof thefirst fourcervical (C1-C4)vertebrae inC. longicollis.
Lateral view
Dorsal view
Frontal view
Caudal view
C5 C6 C7 C8
figure�.� Lateral, dorsal, frontal, and caudal views of the last four cervical (C5-C8) vertebrae in C. longicollis.
3339.indb 167 11/26/07 12:07:47 PM
��� BiologyofTurtles
neuralarchofC1andthebaseoftheskullisligamentous.ThejointissimilarinbothApaloneandChelodina.
JointsC2-1,C3-2,C4-3,C5-4(anteriorpartoftheneck).Thearticulationfacetsofvertebraecomposing these joints are very similar. The vertebral centra are all opisthocoelous and haveananteriorellipticalcotyle,ofwhich the longaxis isorientedvertically inChelodinaandhori-zontallyinApalone,andaposteriorcondylewithenlargedlateralridges.Thearticularfacetsofthezygapophysesof themostcranial jointof thisgroup(C2-1)are lessstronglydeveloped.TheelongatedneuralspineofC2extendsanteriorlybeyond thearticular facetsof theposteriorzyg-apophysesofC1inbothgroups.TheposteriorzygapophysesofC1areclearlyseparated.In themorecaudaljoints,thezygapophysesareelongatedandslightlyelevated(Figure7.3).Thearticularfacetsoftheanteriorzygapophysesareslightlyconcaveandtheiroutlineiskidney-shaped.Theyareoriented in a dorso-medial direction.Theposterior zygapophyseal facets are ventrolaterallyinclined.LateralmobilityisstronglyreducedinApalonecomparedtoChelodinabecauseofthepairedpost-zygapophyses.
Lateral view
Dorsal view
Frontal view
Caudal view
C5 C6 C7 D1C8
figure�.� Lateral,dorsal,frontal,andcaudalviewsofthelastfourcervical(C5-C8)andthefirstdorsalvertebra(D1)inA.spinifera.
Lateral view
Dorsal view
Frontal view
Caudal view
C1 C1́ C2 C4C3
figure�.� Lateral,dorsal,frontal,andcaudalviewsofthefirstfourcervical(C1-C4)vertebraeinA. spi-nifera.C1′istheintercentrumoftheatlas.
3339.indb 168 11/26/07 12:07:49 PM
CervicalAnatomyandFunctioninTurtles ���
JointsC6-5andC7-6.ThecentraljointsC6-5andC7-6areprocoelousinChelodinabutopis-thocoelousinApalone.Inposteriorview,theposteriorcondyleofC5inChelodinahastheshapeofaraindrop.Alternatively,inChelodinathecondyleofC6isalmostspherical.ThecongruentcotyleontheanteriorsideofC6andC7haspronouncedlateralandventralridges.Thearticularfacetsoftheposteriorzygapophysisarewelldevelopedandalsohaveakidney-shapedoutline.Thebilateralposteriorzygapophysealfacetscontacteachothermedially,formingasemicircularorhorseshoe-shapedarticularsurface.Thearticularfacetsoftheanteriorzygapophysesaremoreoval(almostcircular)ascomparedtothemoredistaljoints.InApalone,thearticulatoryfacetsofthepost-zyg-apophysesarepositionedhorizontally.Thepost-zygapophysesthemselvesarenearlyinlinewiththelongaxisofthevertebrae(lessthan6°).
JointC8-7.C8-7isopisthocoelousandtheshapeofitscondyleandcotyleresemblepreviouslydescribedophisthocoelousjointsinbothgroups.ThecotylehasenlargedlateralridgesinChelo-dinaandisfullysplitinApalone.TheposteriorzygapophysisofC7iselongated,inclinedindorsaldirectioninbothgroups(Figure7.4).Thearticularfacetsareclearlydistinguishablefromthoseoftheremainderof thezygapophysesandare laterallyorientedinChelodina.TherathershortbutstronglyelevatedanteriorzygapophysesofC8tendtoinclinedorsomediallyinChelodinabutmorelaterallyinApalone.
JointD1-C8(cervico-dorsal joint).D1-C8 isprocoelous inChelodina. InApalone, this jointlacksacentralarticulation.InChelodina,theratherflattenedcondyleontheposteriorpartofC8isalmostcircular.Thearticularfacetfacescaudo-ventrally.ThecircularcongruentcavityontheanteriorpartofD1isshallowerthanthoseonthemoreanteriorjoints.Theelongateposteriorzyg-apophysisinChelodinaismoreelevatedinthedorsaldirectionthanthatofjointC8-7(Figure7.4).Thearticularfacetsofthezygapophysesaremoreinclinedthanthoseinthepreviouslydescribedjoints.OnC8,thearticularfacetsofbothsidesfuseandareclearlydistinguishablefromthepos-teriorzygapophysis.Theirappearanceisthatofaverticallypositionedcylinder(approximatingaverticalhingejoint).TheanteriorzygapophysesonD1arerathershortandpresentanincreaseddor-somedialinclinationofthearticularfacets.InApalone,thepost-zygapophysesofC8areextremelywelldevelopedandwider than thoseof themoreanteriorly situatedvertebrae (Figure7.6).Thearticulatoryfacetsarestronglyconcave.Thepre-zygapophysesofD1havebeenrearrangedtohori-zontallyorientedcylinders.Onlyventroflexionispossibleatthisjoint.
7.3.3 muSculAture
ThefollowingshortdescriptionofthecervicalmusculatureisbasedontheterminologyofShah(1963)forChelodinaandthatofOgushi(1913)forApalone.Othermorphologicalaccountsonthecervicalmusculatureinturtles(Bojanus,1819;George&Shah,1954,1955;Scanlon,1982)wereconsultedtoclarifythepositionofcertainmuscleswhereneeded.
�.�.�.� Chelodina
m.constrictorcolli.Thisisathinsheet-likemusclewhichcoversthefirsttwothirdsoftheneck.Themusclehasadualorigin:somefibersoriginateonthesquamosalandthedorsalconnectivetissueassociatedwiththeoccipitalspineandothersoriginateontheneuralspinesofC2-C6.Thefibersrunventrallyandinsertmidventrallyonacentralconnectivetissuesheet(medianraphe).
m.retrahenscapitisetcollique(Figure7.7).Thismuscleconsistsofanumberofwell-developedandindividualizedbundlesthatconnectthecarapacetotheneckandhead.ThefirstbundleisthelongestandoriginatesatthelateralaspectofD8andtheadjacentpartofthecarapace.Themusclerunscraniallyandinsertsbymeansofalongtendonatthebasioccipital.AnumberoffibersfromthefirstbundleinsertonthelateralaspectofC1-C5.ThesecondbundleoriginatesatthelevelofD5.ThemusclerunscraniallyandinsertstendinouslyatthelateralaspectofC5.Thethirdbundle
3339.indb 169 11/26/07 12:07:50 PM
��0 BiologyofTurtles
Carapace
Tendon
Bundle 2 Bundle 3
Bundle 1 Tendon
Bundle 4
Bundle 2 *
Bundle 2 *
Bundle 6 Bundle 5
Bundle 7 Bundle 4
m. longissimus cervicis
m. longissimus thoracis
Lateral bundles
Medial bundle
Bundle 3 *
Bundle 3 *
Bundle 1 * Bundle 1 *
Bundle 4 *
Bundle 4 *
Pectoral girdle
*
Skull
m. retrahens capitis collique
figure�.� Schematicrepresentationofthem.retrahenscapitiscollique,them.longissimuscervicis,andthem.longissimusthoracisinC. longicollis.
3339.indb 170 11/26/07 12:07:54 PM
CervicalAnatomyandFunctioninTurtles ���
m. semispinalis
m. testocapitis
m. spinalis cervico-capitis
Medial bundle
Lateral bundle
figure�.� Schematicrepresentationofthem.semispinalis,them.testocapitis,andthem.spinaliscer-vico-capitisinC.longicollis.
3339.indb 171 11/26/07 12:07:55 PM
��� BiologyofTurtles
m. rectus cervicis
m. scalenus complex
m. scalenus I
m. scalenus II
m. scalenus III
m. scalenus IV
Tendon of m. retrahens capitis collique
figure�.� Schematicrepresentationofthem.rectuscervicisandthem.scalenuscomplexinC. longicollis.
3339.indb 172 11/26/07 12:07:56 PM
CervicalAnatomyandFunctioninTurtles ���
m. constrictor hyoideus
mm. intertransversarii colli
m. longus colli
b8 b6
b7 b5
6
5 4 3 2 1
b3
b4 b2
figure�.�0 Schematicrepresentationofthem.constrictorhyoideus,themm.intertransversariicolli,andthem.longuscolliinC.longicollis.
3339.indb 173 11/26/07 12:07:58 PM
��� BiologyofTurtles
originatesatD4and insertsat the lateral aspectofC7.The fourthbundleoriginatesatD3andinsertsatthetransverseprocessofC8.
m.longissimuscervicis(LC,Figure7.7).Thisisasegmentallyarrangedmusclewithamorecomplexarrangement.TheLCofC7originatesonthepre-zygapophysisofC8.Themusclebellyrunsanteriorlyanddividesintoamoremedialandalateralbundle.Thelateralbundleinsertsonthepost-zygapophysisofC6andthemedialonthelateralaspectofC5.TheLC6originatesonthepre-zygapophysisofC7andinsertsonthepost-zygapophysisofC5.TheLC5originatesonthepre-zygapophysisofC6andinsertsonthepost-zygapophysisofC4.TheLC4originatesonthepre-zygapophysisofC5andinsertsmainlyontheneuralarchofC1.Duringthecourseofthemuscle,fibersdivergeandinsertonthepost-zygapophysesofC3andC2aswell.
m.longissimusthoracis(Figure7.7).Thismuscleisresponsibleforlateralflexionoftheposte-riorpartoftheneckandconsistsofonemedialandtwolateralbundles.Allbundlesoriginateattheventralaspectofthecarapace,cranialtotheoriginofthem.testocapitis.ThemedialbundleinsertsatthelateralaspectofC8.ThefirstlateralbundleinsertsbymeansofatendononC6atthelevelofthepost-zygapophysis.ThefibersofthelateralmostbundleruninitiallyventraltothepreviousbundlebutrunmorelaterallyatthelevelofC7.Themusclebundleinsertstendinouslyatthelevelofthepost-zygapophysisofC5.
mm.semispinalis(Figure7.8).Thisisaseriesofshortseriallyarrangedmusclesthatspanadja-centvertebraeandrunfromC2toC8.Themusclesoriginateattheneuralspineofthemoreposte-riorvertebraandrunanterolaterallytoinsertonthepost-zygapophysisoftheanteriorvertebra.
m. testocapitis (Figure7.8). This is a very long muscle that consists of two distinct musclebundlesthatconnectthecarapacetotheheadandneck.Bothbundlesoriginateattheventralaspectofthecarapace,lateraltothescapula.ThefibersofthemedialbundleconvergeontoastrongtendonthatinsertsatthelateralaspectofC5.ThelateralbundleinsertsthroughacomplexarrangementoftendonsintheanteriorpartoftheneckatthelateralaspectofC4-C2.
m.spinaliscervico-capitis(Figure7.8).Thisissuperficialdorsalcervicalmusclebundle.ThemuscleoriginatesattheneuralspinesofC3-C5andrunsobliquelyanteriorly.Themoremediallypositionedfibersinsertonthelateralaspectoftheoccipitalspineandthemorelateralfibersonthedorsalaponeurosiscoveringthem.adductormandibulae.
m.rectuscervicis(Figure7.9).This isaverylongandventrallypositionedmusclethatcon-nectsthepectoralgirdlewiththeventralaspectofthehyobranchium.Themuscleoriginatesatthemid-dorsalaspectofthecoracoid.Thefibersrunventrallyandjointhefibersofthem.constrictorhyoideusofthesameside.Atthelevelofthehyobranchium,thetwomusclesseparateagainandthefibersofthem.rectuscervicisinsertpartiallyonthesecondceratobranchialandpartiallyonthebasibranchium.
m. scalenus complex (Figure7.9).This is a seriesof fourmuscles that connect thepectoralgirdlewiththelateralaspectoftheposteriorneck.Themostsuperficialbundleisverythinandrunsbetweentheacromion,thedorsalaspectoftheplastron,andtheposteriormostcervicalvertebrae(C6-C8).Thesecondandventralmostbundleofthescalenuscomplexoriginatesontheacromionbutdistallytothefirstbundle.ThefibersinsertattheventrolateralaspectofC4andC5.Thethirdbundleoriginatespartlyonthescapulaandpartlyontheacromion.ThefibersconvergeonaflattendonthatinsertsattheventrolateralaspectofC5.Thefourthpartistheshortestandoriginatesacrosstheentirewidthofthescapula.Themuscleinsertsontheproximalaspectofthetendinousinsertionofthesecondbundleofthem.retrahenscapitisetcollique.
m.constrictorhyoideus(Figure7.10).ThismuscleoriginatesatthelateralaspectofC6,runscraniallyandinsertsattheposterioraspectofthefirstceratobranchial.Althoughitsprimaryfunc-tionispresumablyrelatedtotheabductionandretractionoftheceratobranchial,itpresumablyalsoplaysaroleduringlateralbendingoftheneck.
mm. intertransversarii colli (Figure7.10). This is another segmentally arranged muscle thathasadualorigin.Thelateralbundleoriginatesattheventralaspectandthemedialbundleatthe
3339.indb 174 11/26/07 12:07:58 PM
CervicalAnatomyandFunctioninTurtles ���
diapophysisofthecervicalvertebra.Bothbundlesjoinandinsertattheposterioraspectofthever-tebralcentrumofthemorecraniallypositionedvertebra.
m.longuscolli(Figure7.10).Thismuscleconsistsofaseriesofsegmentallyarrangedsmallmusclebundlesthatrunincloseassociationtothem.retrahenscapitisetcollique.Thelonguscollibundlesofthefirstfivevertebraeoriginateattheventralaspectoftherespectivevertebrae.Inser-tiontakesplacebymeansofthetendinousinsertionofthesecondbundleofthem.retrahenscapitisetcolliqueonthelateralaspectofC4-C1.Themusclesinthemoreposteriorpartoftheneckaresomewhatmorecomplex.Them.longuscolli8originatesontheventralsideofC8andinsertsonthetendonofthesecondbundleofthem.retrahenscapitisetcollique.Thelonguscolli7originatesattheventralaspectofC7andinsertsbymeansoftheinsertionofthetendonofthesecondbundleofthem.retrahenscapitisetcolliqueatthelateralaspectofC5.Thelonguscolli6originatesattheventralaspectofC6andinsertsatthelateralaspectofC5.
�.�.�.� Apalone
NotableinApaloneisthestrongreductionoftheepaxialmusculature(Hofstetter&Gasc,1969),theabsenceofthelongissimussystem,andtheabsenceoftheiliocostalissystemthatischaracteristicforallturtles.
m. constrictor colli (m. sphincter colli, Ogushi, 1913). This muscle is poorly developed inApaloneandconsistsoftwodiscreteparts.Theanteriorpartcoversthelateralandventralaspectoftheanteriorneckregion.Itoriginatesonthedorsalconnectivetissueassociatedwiththeoccipitalspineandrunsventrallytoinsertonthemedianraphe.Theposteriorpartisoftendifficulttodis-cernandoriginatesmoreposteriorlyintheneckonthenuchalconnectivetissueassociatedwiththeneuralspinesandinsertsventrallyonthemedianraphe.
m.rectuscervicis(m.coraco-hyoideus,Ogushi,1913).Thisistheventral-mostmuscleinthecervicalregion.Themusclehasadualorigin—onepartoriginatesonthedorsalaspectoftheproxi-malpartoftheprocoracoid,andtheotherpartoriginatesatthedorsalaspectoftheepicoracoid.Themusclerunsanteriorlyandinsertsattheposterioraspectofthebasibranchium.
m.carapaco-basioccipitis (Figure7.11).This isavery longmuscle that runs fromthepelvicgirdletothecranium.Threedistinctoriginsonthecristamedianaventralisofthefirst,second,andthirdcaudalvertebraearepresent.Inaddition,threeaccessorysitesoforigincanbediscerned:ontheproximalaspectofthefifthrib,acrosstheentirelengthofthesixthcostalplate,andalongtheentireseventhribandassociatedcostalplate.Thefibersofthislastbundleinitiallyrunatanangleofatmost90°tothelongaxisoftheremainderofthemuscle.
m. carapaco-basioccipitis
Costal plate Rib
figure�.�� Schematicrepresentationofthem.caracapo-basioccipitisinA. spinifera.
3339.indb 175 11/26/07 12:07:59 PM
��� BiologyofTurtles
A
m. spinalis cervico-capitis - cauda hyoidea
m. spinalis cervico-capitis - cauda squamosi
m. spinalis cervico-capitis - cauda occipitalis
m. spinalis cervico-capitis - cauda cornu branchiale II
m. spinalis cervico-capitis
m. articulo-transversalis longus
B E
C F
D
figure�.�� Schematicrepresentationofthem.spinaliscervico-capitiscomplex(A–D),them.cervico-spinalis(E),andthem.articulo-transversalislongus(F)inA. spinifera.
A
m. articulo-transversalis brevis
m. articulo-cruralis longus
m. intertransversalis
m. transverso-corporis
m. cervico-spinalis lateralis brevis dorsalis
m. epistropheo-squamosus ventralis
B E
C F
D
figure�.�� Schematicrepresentationofthem.articulo-transversalisbrevis,them.articulo-cruralislon-gus,them.intertransversalis,them.transverso-corporis,them.cervico-spinalislateralisbrevisdorsalis,andthem.epistropheo-squamosusventralisinA. spinifera.
3339.indb 176 11/26/07 12:08:02 PM
CervicalAnatomyandFunctioninTurtles ���
A
m. epistropheo-squamosus dorsalis
m. epistropheo-atlantis dorsalis
m. epistropheo-atlantis ventralis
m. atlanto-exoccipitis
m. atlanto-basioccipitis medialis
m. atlanto-opisthoticus
B E
C F
D
figure�.�� Schematicrepresentationofthem.epistropheo-squamosusdorsalis,them.epistropheo-atlan-tisdorsalisandventralis,them.atlanto-exoccipitis,them.atlanto-basioccipitismedialis,andthem.atlanto-opisthoticusinA.spinifera.
A
B m. epistropheo-odontoideus
m. cortico-cervicale I
m. cortico-cervicale II
m. cortico-cervicale III
C
D
figure�.�� Schematic representation of the m. epistropheo-odontoideus and the m. cortico-cervicalegroupinA. spinifera.
3339.indb 177 11/26/07 12:08:04 PM
��� BiologyofTurtles
m. spinalis cervico-capitis (cervico-hyo-capitis, Ogushi, 1913; Figure7.12). This is a largemusclethatconsistsoffourdistinctmusclebellies—thecaudahyoidea, thecaudasquamosi, thecaudaoccipitalis,andthecaudacornubranchialeII.Thecaudahyoidea(Figure7.12A)originatesattheneuralroofofC6,runsanteriorlyandmergeswiththefibersofthem.rectuscervicis.Thecaudasquamosi(Figure7.12B)partoriginatesattheneuralroofofC5,runscranially,andinsertsbymeansofabroadaponeurosisattheposterioraspectofthesquamosum.Thecaudaoccipitalis(Figure7.12C)originatesattheneuralroofofC4,runscranially,andinsertsontheexternalfas-ciacoveringtheexternaladductor.ThecaudacornubranchialeII(Figure7.12D)originatesattheneuralroofofC3,runscranially,andinsertsattheconnectivetissueassociatedwiththetipofthesecondceratobranchial.
m.cervico-spinalismedialis(Figure7.12E).Thisisasegmentallyarrangedmuscle.Themus-clesoriginatetendinouslyattheanterioraspectofthecristamedianaventralis,runanteriorly,andinserttendinouslyattheposterioraspectofthecristamedianaventralisofthethirdmorecraniallypositionedvertebra.ThemusclesoriginatingontheC2-C4mergeandinsertjointlyattheventralaspectofthebasioccipitalbymeansofawell-developedtendon.
m.cervico-spinalislateralislongi(Figure7.12&Figure7.13).Thisconsistsofthreelong,super-ficialmusclesthatoriginateatthelateralaspectofthezygapophysesofC5throughC8:
Them.articulo-transversalislongus(Figure7.12F)originatesonthelateralaspectofthepre-zygapophysesofthelastfivecranialvertebrae.Themusclerunscraniallyacrosstheventralaspectofthetwomorecraniallysituatedvertebraeandinsertsonthetransverseprocessofthefollowingvertebra.Them.articulo-transversalisbrevis (Figure7.13A)runsacross threevertebrae. Itorigi-natesatthedorsalaspectofthepre-zygapophysesandinsertsjointlywiththem.articulo-transversalislongus.Them.articulo-cruralislongus(Figure7.13B)originatestendinouslyatthedorsalaspectof thepre-zygapophyses, runscranially, and insertsdirectlyat thedorsal aspectof thepost-zygapophysesofthethirdmorecraniallypositionedvertebra.
mm.cervico-spinalislateralisbrevesventrales(Figure7.13).Thisconsistsoftwodistinctmus-clegroups,themorelaterallypositionedmm.intertransversales(Figure7.13C)andthemoremedi-allypositionedmm.transverso-corporis(Figure7.13D).Theformeroriginatestendinouslyatthetransverseprocess,runscranially,andinsertsdirectlyontheposterolateralaspectofthepre-zyg-apophysisandtheposterioraspectofthetransverseprocessofthemorecraniallysituatedvertebra.Them.transverso-corporisoriginatesontheventralaspectofthetransverseprocessandtheverte-bralbody,runscranially,andinsertsonthecondyleofthemorecraniallysituatedvertebra.
mm.cervico-spinalis lateralisbrevisdorsales(Figure7.13E).This isasegmentallyarrangedmusclerunningfromC7toC3.Eachsegmentconsistsoftwodistinctparts.Themedialpartorigi-natesatthelateralaspectofthecristalateralisofthepost-zygapophysisandinsertsatthedorsalaspectofthebaseofthepost-zygapophysesonthemorecraniallypositionedvertebra.Thelateralpartoriginatestendinouslyontheconnectivetissuesurroundingthezygapophysealarticulationandinsertsatthedorsolateralaspectofthepost-zygapophysisofthemorecraniallysituatedvertebra.
m.epistropheo-squamosusventralis (Figure7.13F).Themuscleoriginatesaponeuroticallyattheposterioraspectofthecristamedianaventralisoftheaxis.Themusclerunsanterodorsallytoinsertattheprocessusmastoideus(Ogushi,1913)ofthesquamosal.
m. epistropheo-squamosus dorsalis (Figure7.14A). This originates fleshy at the neural archandtheposterioraspectoftheneuralroofoftheaxis.Themusclerunsanteriorlyandinsertsattheprocessusmastoideusofthesquamosalandtheposterioredgeoftheopisthoticum.
m.epistropheo-atlantisdorsalis(Figure7.14B).Thisoriginatesattheanterioraspectoftheneu-ralarchoftheaxis.Themusclerunsanteriorlyandinsertstendinouslyattheprocessusarticularistransversalisventralisoftheatlas.
•
•
•
3339.indb 178 11/26/07 12:08:05 PM
CervicalAnatomyandFunctioninTurtles ���
m.epistropheo-atlantisventralis(Figure7.14C).Thisliesventraltothepreviousmuscle.Itorig-inatesfleshyatthelateralaspectoftheaxis,bothdorsalandventraltothetransverseprocess,andinsertsbymeansofashortaponeurosisattheprocessusarticularistransversalisoftheatlas.
m.atlanto-exoccipitis(Figure7.14D).Thisoriginatesatthetendinousinsertionofthem.rectuslateralisontheatlasandinsertsfleshyattheexoccipital.
m.atlanto-basioccipitismedialis(Figure7.14E).Thisoriginatesbymeansofashorttendonattheprocessusarticularisventralisoftheatlas.Themusclerunscraniallyanddivergestowarditsoriginonthebasioccipital.
m.atlanto-opisthoticus(Figure7.14F).Thisoriginatesatthedorsolateralaspectoftheatlasandinsertsatthedorsalsurfaceoftheopisthoticum.
m.epistropheo-odontoideus(Figure7.15A).Thisoriginatesatthelateralaspectoftheatlas,justdorsaltothecristamedianaventralis,andinsertsbymeansofanarrowtendonattheprocessusodontoideus.
m.cortico-cervicaleI(Figure7.15B).Thisoriginatesfleshyattheanteromedialaspectofthenuchalplateandinsertsmusculouslyatthelateralaspectofthepost-zygapophysisofC6.
m. cortico-cervicale II (Figure7.15C). This originates at the nuchal plate, just posterior totheoriginof them.cortico-cervicale I.Themuscle insertsfleshyat theneural roofofC6, justposteriortotheinsertionofthecaudahyoideaofthem.cervico-hyo-capitis.
m. cortico-cervicale III (Figure7.15D). This has adualorigin.The lateralpartof themuscleoriginatesatthelateralaspectoftheventralsideofthenuchalplatebymeansofanarrowandlongmuscularhead.Themedialheadoriginatesposteriortotheoriginofthem.cortico-cervicaleII.Bothheadsmergetowardtheoriginontheneural roofofC7, lateral to the insertionof them.cer-vico-spinalislateralisbrevisdorsalis.
m.spinalisdorso-lumbalis.Thissegmentallyarrangedmuscleispositionedinthecanaliscollateralisvertebralis(Vallois,1922).Theoriginispartlyontheinneraspectofthecanalandpartlyonthedorsalaspectofthecapitulumoftherib.Themuscleleavesthecanaliscollateralisver-tebralisrightposteriortotheribassociatedwithD1andinsertsdirectlyatthepost-zygapophysisofC8.
7.3.4 neckmovementS
NeckmovementsinChelodinaaredescribedindetailbyVanDammeetal.(1995,2002),Aertsetal.(2001),Weis-gram et al. (1992), and Van Damme and Aerts (1997).PreviouslyunpublishedinformationonneckmovementsinApaloneandChelodinaarepresentedhere.
�.�.�.� neckretractionandCer�icalmobilityinApalone ferox
Intheextendedconfiguration,jointsC9-8,C8-7,andC7-6showthelargestinitialangles.Themorecraniallyposi-tioned joints are all in the extended configuration. TheretractionoftheneckinApaloneischaracterizedbyrela-tivelysmallangularchangesinthefirstthree(C3-2,C2-1,andS-C1)andthelastcervicaljoint(C9-8).Thelargest
A
B
C
figure�.�� Static cineradiographsrecorded in dorso-ventral view, showingtheconfigurationofthecervicalvertebraein(A)fullyextended,(B)relaxed,and(C)fullyretractedpositionsof theneckinA. spinifera.
3339.indb 179 11/26/07 12:08:06 PM
��0 BiologyofTurtles
angularchangesduringretractionoccur in themiddleregionoftheneck.JointsC8-7andC7-6extendduringthecourseoftheretraction.ThesituationforjointsC6-5,C5-4,andC4-3ismorecomplex.C6-5initiallyflexesduringtheretrac-tionbutextendsagaintowardthelatterpartofneckretraction.Thetwomorecraniallysituatedjointsstartwithaninitiallyextendedconfigura-tion.Duringretraction,theangleofthesejointsgraduallyincreases.
The potential range of movement in thedorso-ventraldirectionisrelativelylargeinmostvertebral joints but largest in joints C8-7, C5-4, C4-3, and C3-2. However, joint angles dur-ingasimulatedsnorkelingmovementaremostpronouncedatthemostposteriorcervicaljoints(D1-C8,C8-7,andC7-6).Theanteriorjointsarekept relatively constant throughout the move-ments, as is observed during neck retraction(Figure7.16 and Figure7.17). Lateral mobility
oftheneckinA. spiniferaisalsosurprisinglyhigh,withthemoreproximaljointsbeingtheonesallowingmostmovement in thehorizontalplane. Incon-trast, thelastthreecervicaljointsallowalmostnolateralmovements.
�.�.�.� KinematicsofsnorkelinginC. longicollis
Neck movements during snorkeling in C. longicollis aremuch slower in comparison with those involved duringfeeding behavior (Aerts et al., 2001; Van Damme et al.,2002) and escape retraction (Van Damme et al., 1995).The total duration of a typical ventroflexion followed byadorsiflexionisabout6s.Eachcomponentofthemove-ment takes about 3 s. The representative example of asnorkeling movement in C. longicollis is represented bysuccessivestickdiagramsinFigure7.19.Startingfromanextendedconfigurationinwhichheadandneckareslightlydepressed, the animal initially balances its neck aroundthis configuration.This configuration is characterizedbyverysmall startingangles inall joints (withexceptionofD1-C8). Minor changes in joint angles occur during thefirstphaseofthemovementmainlyintheposteriorpartoftheneck(Figure7.20).Duringthisphase,noconspicuouschangesoftheelevationoftheheadandheadpositionareobserved. After approximately 3 s, a conspicuous, rathersteady increase in the elevation of the head is observed,mainlyasaresultofchangesintheangleofjointsC4-3toC7-6.InpatternsobservedinC8-7andD1-C8inthemostposteriorpartoftheneckandS-C1andC2-1inthemostanteriorpartoftheneck,changesinvertebral jointangle
A
B
figure�.�� (A) Static cineradio-graphs recorded in dorso-ventral viewillustratingthepositionandshapeofthecervical vertebrae inC. longicollis. (B)LateralviewofthecervicalsysteminC. longicollis during snorkeling. Note theradio-opaquemarkers insertedonto thecervicalvertebraetofacilitatetheanaly-sesofvertebralmovements.
A
B
C
figure�.�� CT-scan showing the configurationof the neck and the cervical vertebrae of A. spi-niferainfullyextended,relaxed,andfullyretractedpositions.
3339.indb 180 11/26/07 12:08:08 PM
CervicalAnatomyandFunctioninTurtles ���
appear less conspicuously related to the observed movements,showlessoverallchange,anddisplayagenerallymoreirregularmovementpattern.Atveryhighdegreesofelevation—atwhichalso head position is also starting to change—changes in jointanglearemainlyoccurringin jointsC5-4,C6-7,andespeciallyC4-3(morethan30°ofdorsiflexion).ThisisnotthecaseforC6-5.Thisjointreachesupto20°ofdorsiflexionandthenlevelsoff.Figure7.21illustratestheabsoluterangeofmovement(expressedin degrees of flexion) of each joint during the whole sequence.Notice therelativelyminor jointrotations in theanterior(S-C1,C2-1, andC3-2) andposterior parts (C8-7,D1-C8)of theneck.Asmentionedpreviously,themostconspicuouschangesinjointangleareobservedinthemiddlepartoftheneck,especiallyinjointsC4-3,C5-4,andC7-6.
In summary, the range of mobility in the vertical plane ofthecervical joints is limited,especially incomparisonwith thechangesofjointangleinthehorizontalplane.Dorsiflexionofthejoints is more conspicuous than ventroflexion. Ventroflexion inthe joints isnearlynon-existent.Thedominantchanges in jointangleareobservedinthemiddlepartoftheneck.
�.� disCussion
7.4.1 verteBrAlStructure
Thenatureofretractionofthehead-necksysteminturtles(intheverticalplaneasinApaloneorinthehorizontalplanasinChelo-dina)hasprofoundimplicationsonthemorphologyofthecervi-cal system.Although inboth species thecervicalvertebraearemarkedlyelongated,inChelodinathevertebraearerathernarrowand tall. However, in Apalone the situation is reversed and thevertebraearewideandrathershallow.AnothermarkeddifferenceisthepresenceofapronouncedventralvertebralcrestinChelo-dina.InApalone,thesecrestsareonlypresentinthemostanteriorvertebrae.The transverseprocesses that serveas insertion sitesforthemusclesofthelongissimusarewelldevelopedinChelodinaandarepositionedlaterallyattheborderbetweentheneuralroofandthevertebralcentrum.Becausethelongissimussystemisabsent inApalone, the transverseprocessesarepoorlydevelopedandpositionedmorecranially.Also,thestructureoftheposteriorzygapophysesismarkedlydifferentbetweenthetwospecies.WhereasinChelodinathereisonlyasinglebasalelementcarryingthetwohorizontallyorientedarticularfacets,inApalonetwoseparatepost-zygapophysesarepresent.Additionally,thearticularfacetsaremuchmorecurvedinApalonecomparedtothoseinChelodina.However,inbothspeciesthestructureofthezygapophyseslimitsventroflexionconsiderably.
7.4.2 cervIcAlmuSculAture
Thedifferencesinretractionmodeinthetwogroupsarealsoreflectedinthestructureanddifferen-tiationofthecervicalmusculature.Asmentionedpreviously,forexample,thelongissimussystemappearstobecompletelyabsentinApalone.Ashead-neckmovementstypicallyoccurintheverti-calplaneintheseanimals,thisisnotunexpected.Alternatively,inChelodinathelongissimussys-temisstronglydeveloped.Notableinthecryptodiresisthecortico-cervicalmuscularsystemthat
A
B
B ∆T = 0.25 sec.
A
C
Ventroflexion
Horizontal extension
Breathing
Carapace
Dire
ctio
n of
mov
emen
t
figure�.�� (A) Schematicillustration of the neck configu-rationinC. longicollis.(B)Stickdiagram representing an actualsnorkeling movement in C. longicollis.
3339.indb 181 11/26/07 12:08:10 PM
��� BiologyofTurtles
50
40
30
Deg
rees
20
10
0S-C1 C2-1 C3-2 C4-3
Joint
Absolute Range of Movement
C5-4 C6-5 C7-6 C8-7 D1-C8
figure�.�� MovementrangeofthecervicaljointsinC. longicollisintheverticalplane.
80 40
0 –40
40 20
0
20
0
20
0
10
20 10 0
–10
–10
0
8 Head position
Elevation
S−C1
C2−1
C3−2
C4−3
C5−4
C6−5
C7−6
C8−7
D1−C8
4
0 40
20
0
40
20
0
40
20
0
40
20
0.0 1.0 2.0 Time (s)
3.0 4.0 5.0 6.0 0.0 1.0 2.0 Time (s)
3.0 4.0 5.0 6.0
0
0 –40
80 40
0 –40
80 40
0 –40
80
Deg
rees
Join
t Ang
le (°
)
40 0
–40 80 40
0 –40
40 0
–40
40
0
–40
0 –20
–60 –40
0
–40
40
figure�.�0 Kinematicplotsillustratingtimeprofilesofjointangle(dashedcurves,rightverticalaxis)andelevationanglewithrespecttothehorizontalofthemoredistalofthesegmentsconstitutingthejoint(solidcurve,leftverticalaxis)duringasnorkelingmovementinC. longicollis.Thefirstpanelrepresentselevationoftheheadsegmentandtherectilineardistance(rightaxisincm)betweenheadandcarapace.
3339.indb 182 11/26/07 12:08:13 PM
CervicalAnatomyandFunctioninTurtles ���
presumablyfunctionstoprotractandretracttheneck.PreliminaryelectromyographicdataforthecryptodireT. scripta elegans(VanDamme,unpublished)supportthishypothesis.InpleurodireslikeChelodina,thissystemisabsent.Ratherunexpectedly,them.sphinctercolliispoorlydevelopedinApalone.InChelodinaandmostotherpleurodires,thismuscleappearsmuchbetterdevelopedandmayactuallyplayaroleinaligningthecervicalmusclebundlesalongthecervicalcolumnduringneckmovements.However,thisremainsspeculativeandmustbetestedbyelectromyography.Also,thedominantheadretractormuscle,them.retrahenscapitisetcolliqueinChelodina(Shah,1963)andthem.carapace-basioccipitisinApalone(Ogushi,1913),isnotablydifferentinthetwospecies.InApalone,thismuscleconsistsofapairedmassivemusclebundlerunningfromthefirstcaudalvertebratothebackoftheskull.InChelodina,thismuscleismorecomplexandconsistsofseveraldiscretebundlesthatinsertonspecificsitesalongthecervicalcolumn.
However,astrikingsimilarityamongthetwogroupsistheextremelengthoftheheadretrac-tormuscle.Presumably,elongationoftheretractormuscleallowsformoresarcomerestobeplacedinseries,whichmayconsiderablyincreasethecontractionspeedofthemuscle(Josephson,1975).Moreover, by increasing the number of sarcomeres in series, each individual sarcomere has tocontract less and may thus potentially operate continuously on the plateau of its length-tensionrelationship.However,foranextremelyelongatedmuscletocontractefficiently,allpartsmustcon-tractsimultaneously.Thepolyneural,polysegmentalinnervationofthem.carapaco-basioccipitisin turtles (Guthe,1981)mayallowfor this.Unexpectedly, them.carapace-basioccipitisconsistsnotonlyoffasttwitchfibersbutalsohasaconsiderablepopulationoftonicfibers(Guthe,1981),whichmayhelpcontrolthepositionoftheheadandneckduringslowmovementsornear-stationarybehaviorssuchassnorkeling.
7.4.3 movementpAtternS
Thecranio-cervicalsysteminturtlescanbecharacterizedasanopenkinematicchainofeightver-tebraeandninejoints.Becauseeachjointtheoreticallyhasthreerotationaldegreesoffreedom(andtoalimitedextentalsothreetranslationaldegreesoffreedom),andbecausethepositioningofthevertebraemustbeachievedbythecombinedactionsofroughly50symmetricalmusclebundles,thecontrolofthepositionoftheheadinspaceobviouslypresentsachallengingcontrolproblem(Aertsetal.,2001).However,thecontrolofthesystemislargelysimplifiedbyanumberofmorphologicalconstraintslimitingthemobilityateachjoint.Forexample,inChelodinathehorizontalnatureofthezygapophysealarticularfacetsandtheverticalorientationofthearticularfacetsofthecondyleandthevertebralcentrumwillfacilitatemovementsinthehorizontalplane.Additionalsimplifica-tionofthecontroloftheneckinChelodinaisachievedbythepresenceofdistinctareasofrota-tionalongtheneck(VanDammeetal.,1995).Forinstance,thejointbetweencervicalvertebrae7and8andthejointsbetweencervicalvertebrae6-5and5-4typicallyshowthegreatestrangeofangularchange.Moreover, therestingpositionoftheneckischaracterizedbyadistinctbendattheselocations,facilitatingthecorrectretractionoftheneckusingonlyasimpleactivationofthem.retrahenscapitisetcollique(Aertsetal.,2001).Dorso-ventralmobilityisgreatestatthejointsC3-2throughC6-5.Thejointssurroundingthebi-convexarticularcentrumofC5showthegreatestangularchangeduringdorsiflexion.
ThecervicalsysteminApaloneisnotablydivergentandthekinematicpatternsarealsomark-edlydifferentfromthoseobservedinChelodina.Uponretractionoftheneck,themorecaudallypositionedvertebraearethefirsttostarttheirrotationaroundatransverseaxis.Theangularchangesin all vertebrae arenegativeduring retraction, suggesting that theneck is retractedas a safety-linked bicycle chain. Cervical vertebrae 5, 6, and 7 undergo an angular change of nearly 180°,whichimpliesthatthesevertebraeendupwiththeirventralaspectalongsidetheventralaspectoftheimmobiledorsalvertebrae.Thisismadepossiblebythenearlycompletereductionoftheventralcrestofthesevertebrae.ItisalsostrikingthatC8remainsinanear-verticalpositionatthemomentoffullprotraction.Themoreanteriorvertebrae(C1toC4)donotcontributetoneckretractionat
3339.indb 183 11/26/07 12:08:13 PM
��� BiologyofTurtles
all.Thebicycle-chain-likeretractionpatternobservedforApaloneisincontrasttotheretractionpatternssuggestedinothercryptodiressuchasTestudoorTrachemys(Scanlon,1982;Weisgram&Splechtna,1990).Inthelatterspecies,aswellasinChelodina,distinctrotationcentersarepresentwherethemajorityoftheangularrotationtakesplace.Thus,theextremelyelongatedneckandasso-ciatedmorphologyofApalonemaynotberepresentativeforthecryptodiranconditioningeneralbutprovideanexcellentexampleofahighlyspecializedcervicalsystemthatcanbecomparedtotheconditioninChelodina.Clearly,furtherinvestigationintothemorphologyandfunctionofthecervicalsysteminturtlesisneededtoincreaseourunderstandingoftheevolutionofthecervicalsystemanditscontrolinturtles.Especiallyinsightfulwouldbestudiesexploringcervicalstructureandfunctioninabroadersampleofturtlesincludingshort-neckedrepresentativesofbothcrypto-diresandpleurodires.Functionalapproachesincludingelectromyographyofthecervicalmuscles,albeitchallenging,areessentialtofurtherourunderstandingoftheevolutionofthecervicalsysteminturtles.
aCKnoWledgments
WethankA.DeSchepper(UniversityHospitalAntwerp)forallowingustousethecineradiographymachineandCTscanner;theAntwerpZooforprovidinguswiththespecimensofChelodina lon-gicollis;G.A.Wood(Wood,1982)forprovidinguswithasoftwarepackagefordatasmoothinganddifferentiation;andA.CuylitsformakingthedrawingsofthecervicalvertebraeinC. longicollis.ThisstudywassupportedbyIWONL-grant910091(JV)andFKFO-grant2.9005.90(FDV).AHisapostdoctoralfellowofthefundforscientificresearch,Flanders,Belgium(FWO-Vl).
referenCes
Aerts,P.,VanDamme,J.,andHerrel,A.,Intrinsicmechanicsandcontroloffastcraniocervicalmovementsinaquaticfeedingturtles,Am. Zool.,41(6),129–1310.2001.
Bojanus,L.H.,Anatome Testudinis Europaeae, Vol. 1.,Vilnae,Luthuania, republished in1970,FacsimilereprintsinHerpetology,no.26,SocietyfortheStudyofAmphibiansandReptiles,Ohio,1819.
Bojanus,L.H.,Anatome Testudinis Europaeae, Vol. 2.,Vilnae,Luthuania, republished in1970,FacsimilereprintsinHerpetology,no.26,SocietyfortheStudyofAmphibiansandReptiles,Ohio,1821.
Dalrymple,G.H.,IntraspecificvariationinthecranialfeedingmechanismofturtlesofthegenusTrionyx,J. Herpetol.,11,255–285,1977.
Deban,S.M.,Wake,D.B.,andRoth,G.,Salamanderwithaballistictongue,Nature,389,27–28.1997.Ernst,C.H.,andBarbour,R.W.,Turtles of the World,Washington,DC:SmithsonianInstitutionPress,1989.Gans,C.,Whydevelopaneck?,inThe Head-Neck Sensory Motor System,A.Berthoz,P.Vidal,andW.Graf
(eds.),NewYork:OxfordUniversityPress,1992,17–21.George,J.C.,andShah,R.V.,ThemyologyoftheheadandneckofthecommonIndianpondturtle,Lissemys
punctatagranosaSchoepff,J. Anim. Morph.,1,1–12,1954.George,J.C.,andShah,R.V.,ThemyologyoftheheadandneckoftheIndiantortoise,Testudo elegans,J.
Anim. Morph.Physiol.,2,1–13,1955.Guthe,K.F.,Reptilianmuscle:Finestructureandphysiologicalparameters,inBiology of the Reptilia, Vol 1,
C.GansandT.Parsons(eds.),NewYork:AcademicPress,1981,265–354.Heidweiller,J.,VanDerLeeuw,A.H.J.,andZweers,G.A.,Cervicalkinematicsduringdrinkingindeveloping
chickens,J.Exp. Zool.,262,135–153,1991.Herrel,A.,Meyers,J.J.,Nishikawa,K.C.,andAerts,P.,Themechanicsofpreyprehensioninchameleons,J.
Exp. Biol.,203,3255–3263,2000.Hofstetter,R.,andGasc,J.P.,Vertebraeandribsofmodernreptiles,inBiology of Reptilia, Vol. 1,C.Gansand
T.Parsons(ed.),NewYork:AcademicPress,1969,201–231.Irschick,D.J.,andGarland,T.Jr.,Integratingfunctionandecologyinstudiesofadaptation:Investigationsof
locomotorcapacityasamodelsystem,Ann. Rev. Ecol. System.,32,367–396,2001.Josephson,R.K.,Extensive and intensive factors determining theperformanceof striatedmuscle, J. Exp.
Biol.,194,135–154,1975.King,G.,Reptiles and Herbivory,London:Chapman&Hall,1996,160.
3339.indb 184 11/26/07 12:08:14 PM
CervicalAnatomyandFunctioninTurtles ���
Ogushi,K.,AnatomischeStudienander japanischendreikralligenLippenschildkröte (Trionyx japonicus):Muskelundperipheresnervensystem,Morph. Jb.,46,299–562,1913.
Pritchard,P.C.H.,Piscivoryinturtles,andevolutionofthelongneckedChelidae,Symp. Zool. Soc. Lond.,52,87–110,1984.
Romer,A.S.,andParsons,T.S.,The Vertebrate Body,Philadelphia:W.B.Saunders,1977,161–167.Scanlon,T.C.,AnatomyoftheneckoftheWesternPaintedturtle(Chrysemys picta belliGray;Reptilia,Tes-
tudinata),unpublisheddiss.,UniversityofMichigan,UniversityMicrofilmsInternational,1982.Shah,R.V.,TheneckmusculatureofaCryptodire(Deirochelys)andaPleurodire(Chelodina)compared,Bull.
Mus. Comp. Zool.,129,343–368,1963.Vaillant,M.L.,Mémoiresurladispositiondesvertebrescervicaleschezleschéloniens,Ann. Sci. Nat. Zool.
Paleont.,Ser.6,10,1–106,1881.Vallois,H.V.,Lestransformationsdelamusculaturedel’épisomechezlesvertébrés,Arch. Morph. Gen. Exp.,
13,180–217,1922.VanDamme,J.,andAerts,P.,KinematicsandfunctionalmorphologyinaquaticfeedinginAustraliansnake-
neckedturtles(Pleurodira;Chelodina),J. Morph.,233,113–125,1997.VanDamme,J.,Aerts,P.,andDeVree,F.,Kinematicsoftheescapeheadretractioninthecommonsnake-necked
turtle,Chelodina longicollis(Testudines:Pleurodira:Chelidae),Belg. J. Zool.,125,215–235,1995.VanDamme,J.,andAerts,P.,CervicalmovementsduringpreycaptureinAustraliansnakeneckedturtles,
genusChelodina(Pleurodira,Chelidae),inTopics in Functional and Ecological Vertebrate Morphol-ogy, A Tribute to Frits De Vree,P.Aerts,K.D’Août,A.Herrel,andR.VanDamme(eds.),ShakerPub-lishingBV,2002,77–94.
Weisgram,J.,andSplechtna,H.,Intervertebralmobilityintheneckoftwoturtlespecies(Testudo hermanni hermanni,Pelomedusa subrufa),Zool. Jb. Anat.,120,425–431,1990.
Weisgram,J.,andSplechtna,H.,CervicalmovementsduringfeedinginChelodina novaeguinaeae(Chelonia,Pleurodira).Zool. Jb. Anat.,122,331–337,1992.
Williams,E.E.,Variationandselectioninthecervicalcentrearticulationsoflivingturtles,Bull. Amer. Mus. Nat. Hist.,94(9),505–562,1950.
Wood,G.A.,Datasmoothinganddifferentiationproceduresinbiomechanics,inExercise and Sports Sciences Reviews,10,308–362,1982.
Wren,K.,Claussen,D.L.,andKurz,M.,Theeffectsofbodysizeandextrinsicmassonthelocomotionoftheornateboxturtle,Terrapene ornata,J. Herpetol.,32,144–150,1998.
Zani,P.A.,Gottschall,J.S.,andKram,R.,Gianttortoiseswalkwithoutinvertedpendulummechanical-energyexchange,J.Exp. Biol.,208,1489–1494,2005.
3339.indb 185 11/26/07 12:08:14 PM
���
8 FunctionalEvolutionofFeedingBehaviorinTurtles
Vincent Bels, Sabine Baussart, John Davenport, Marc Shorten, Ruth M. O’Riordan, Sabine Renous, and Julia L. Davenport
Contents
8.1 Introduction......................................................................................................................... 1878.2 Objective............................................................................................................................. 1888.3 FeedingStructures.............................................................................................................. 1898.4 OverviewofFeedingandDrinkingBehaviors................................................................... 1898.5 Kinematics.......................................................................................................................... 192
8.5.1 TerrestrialFeeding................................................................................................... 1928.5.1.1 Ingestion..................................................................................................... 1928.5.1.2 Intra-oralTransportCycle.......................................................................... 1958.5.1.3 Swallowing................................................................................................. 197
8.5.2 AquaticFeeding....................................................................................................... 1988.5.2.1 IngestionCycle........................................................................................... 1988.5.2.2 ManipulationandTransportCycle.............................................................204
8.5.3 FeedinginDermochelys coriacea:ATypicalExampleofaMarineTurtlewithaHighlySpecializedDiet........................................................................................2048.5.3.1 MaterialsandMethods...............................................................................2048.5.3.2 Results........................................................................................................204
8.6 EvolutionofFeedingBehaviorinTurtles...........................................................................2088.6.1 Ingestion...................................................................................................................2088.6.2 Transport(andOtherFeedingPhases).....................................................................208
Acknowledgments.......................................................................................................................... 210References...................................................................................................................................... 210
�.� introduCtion
Turtleshaveoneofthemostunusualbodyplansamongtheamniotes,withacarapacethatconstrainstheirbehavioralactivitiesdifferentlyfromallothertetrapods(Gaffney,1979;Reisz&Laurin,1991;Laurin&Reisz,1995;Lee,1997;Schafferetal.,1997;Poughetal.,2001;Cebras-Thomas,2005;Kear&Lee,2006;Shedlocketal.,2007).Althoughtheoriginandevolutionofthiscladeofverte-bratesisstilluncleardespitethoroughdiscussion(Joyce&Gauthier,2004;Hill,2005;Nagashimaetal.,2005),suchabodyplanappearstobeconservative.However,reductionoftheshellhasbeenreportedinrelationtochelonianlifestrategies,especiallyinaquaticturtles(Pritchard,1979).Evo-lutionarytransformationsofthelocomotorsysteminaquaticturtlesenablethemtodisplayhighlyadaptedmobilityandmaneuvering,presumablyforoptimizingsearchingforfood,sexualpartners,andareasforegglaying(Davenport&Clough,1986;Renous&Bels,1989;Wyneken,1997;Chap-ter5).Althoughsomeseaturtlepopulationsnestandfeedinthesamegeneralareas,others(speciesorpopulationswithinaspecies)canmigrateovergreatdistances.Leatherbackshaveprobablythe
3339.indb 187 11/26/07 12:08:15 PM
��� BiologyofTurtles
longestmigrationofallseaturtlesbecausetheycanbefoundmorethan7000kmfromtheirnestingbeaches(Hughesetal.,1998).
Despitetheir“armoredtank”shape,turtleshaveadoptedspecializedforagingandfeedingstrat-egiesderivedfromthoseofthestemreptilesthathaveprovidedenduringadvantageswhilesimulta-neouslyimposingbasicrestrictions.Thelivingspeciesofturtles,distributedamong99generaand14families(Ernst&Barbour,1989),inhabitalargenumberofvariousecologicalnichesinmarine,freshwater,andterrestrialhabitatsfromtemperateandtropicalregionsofallcontinentsexceptAnt-arctica,andinalloceans.Itiswellknownthatturtlesareeitherspecializedorgeneralizedintheirdiet,withpossibleontogenicdietaryshiftsbeingcommon(Pritchard,1979).Foragingandfeedingbehaviorshavebeengenerallydescribedforalmostallfamiliesofturtles(Pritchard,1979).Dietarypreferencesandontogenicchangesduringthelifecyclesofturtleshavebeenreportedinnumer-ousspecies,particularlyinmarineturtles.Mostcheloniansshowdietaryfluctuationsduringtheirlivesandcanshiftfromcarnivoroustoomnivorousandherbivorousdietsastheyage(Harless&Morloch,1979;McCauley&Bjorndal,1999;Bouchard&Bjorndal,2006).Briefly,trueterrestrialturtles(Testudinidae)aretheonlyspeciesabletoingestandswallowfoodonland,soaresubjecttothesameconstraintsasallothertrulyterrestrialvertebrates.Apartfromtheseturtles,alargenum-berofspeciesmakeforaysintoterrestrialhabitatsforfeedinganddrinking(e.g.,Emydidae).Someotherspeciesarewhollywater-feedersandchasetheirfoodbyusingvariousstrategiesfromactiveforagingtosit-and-waitpredatorbehavior(e.g.,softshells,Tryonichidae).Somespeciescancapturelivefoodonlandbutswallowitinwater.Severalherbivorousspecies(e.g.,Bataguridae)canfeedonplantmaterialatthesurfaceofthewaterorcandragterrestrialvegetationintothewater(Davenportetal.,1992).Somespeciescanfeedonlandorinwater,completingtheprocessfromfoodcapturetoswallowingineithermedium(e.g.,Terrapene carolina,Emydidae).Althoughbaskingonlandhasbeenreportedinafewcases,marineturtlesaregenerallyfoundonlandonlyintwosituations:whenadultfemalesreturntothelandforegglayingandwhenhatchlingsmusttravelfromthenestdownthebeachtothesea.Marineturtlesalwaysforageandfeedinthewater.
Aseriesofphylogeneticstudieshavedemonstratedsignificantrelationshipsbetweenvariouscharacteristicsofthetrophicsystemandcorrespondingecologicalspecializationsinsomegroupsof turtles (Gaffney, 1975, 1979; Claude et al., 2004). From these data, there have been severalhypothesesgenerated todemonstrate a relationshipbetween feedingperformancesand thephy-logenetichypotheses.Forexample,themorphologicalvariationofthesuperfamilyTestudinoidearelatestobothdietandhabitat(Claudeetal.,2004).Theseauthorssuggestthataspectsofthefeed-ingmode(e.g.,diet)canbeakeyfactorindeterminingmorphologicalevolutionanddiversificationofturtleskulls.Phylogenyappearstoconstrainonlylocalizedfeaturesoftheskullandremainsofminorinfluence.
�.� oBJeCtive
Therearesubstantialproblemsinprovidingacompleteoverviewofthefeedingbehaviorofturtlesusingaquaticandterrestrialhabitatsbasedonlyonpreviouslypublisheddata:kinematicandfunc-tionalanalysesofcompletefeedingmechanismsarerelativelyrare,andfeedingbehaviorhasbeenstudiedusingnon-standardizedmethodsandtechniques.Inhiscomprehensivevolumeofevolutionoffeedingbehaviorintetrapods,Schwenk(2000)providesanexhaustivelistofliteraturedescrib-ingfeedingbehaviorandmechanismsinturtles.Thepresentchapterprovidesthefirstcomparativeanalysisoffeedingbehaviorinturtles.Thoughaquaticfeedinghasbeenstudiedinseveralverydif-ferentrepresentativespeciesdrawnfromfreshwaterfamilies,fewdataareavailableconcerningthefeedingbehaviorofmarineturtlesorofterrestrialturtles.Consequently,thischapterwillnotonlyprovideinformationfromtheliteraturebutwillalsopresentdatacollectedbythechapter’sauthors,togetherwiththeiranalysesoffeedingbehaviorinterrestrialandaquaticspecies.Allofthesedataareconsideredfromacomparativeviewpoint.Whennecessary,arapidsurveyofthemethodsused
3339.indb 188 11/26/07 12:08:16 PM
FunctionalEvolutionofFeedingBehaviorinTurtles ���
forcollectingdataisprovidedforeachofthefeedingmodes,terrestrialandaquatic.Themecha-nismofneckmovementsthatplayakeyroleinfeedinginturtlesisdiscussedinChapter7.
�.� feedingstruCtures
Detailedanalyseshavebeenmadeoftheskull,hyobranchium,andtongueinturtles(Lindeman,2000;Schwenk,2000;Claudeetal.,2004).Whenneeded,weprovidemorphologicaldatathatarenecessary to explain the functional andbiomechanical aspectsof feedingbehavior.Some func-tionalanalysesdemonstratehowstructuresareusedinvariousphasesoffeedinginaquaticturtles(Schwenk,2000;Lemelletal.,2002).
�.� overvieWoffeedinganddrinKingBehaviors
Arapidsurveyoftheevolutionofturtlesandtheirdietshowthatturtlesusetwomainfeedingstrate-giestogainwater,energy,andnutrients:feeding/drinkingonlandandfeedinginwater.Drinkingwaterisaparticularproblemforterrestrialturtlesandforsometurtleslivinginsaline/marinehabi-tats.Alloftheseturtlesneedtodrinkwater(whenavailable)tomaintainthehydricbalancethatisessentialtowelfareandsurvival.Waterintakeratesandfrequenciesarehighlyvariableamongturtles.Forexample,somedesertturtlesareabletoconsumealargeamountofwaterinoneintake,therebyincreasingbodymassbyupto40%.Drinkinginaquaticturtleshasbeenrarelydescribed.Arecentstudydemonstratedthathatchlingseaturtlescanosmoregulateeffectivelyandgainmassbydrinkingseawater(Reinaetal.,2002).Althoughwellreportedintheliterature,drinkingbehav-iorhasbeenquantifiedonlyinMalaclemys terrapin(Davenport&Macedo,1990;Belsetal.,1995).Thisspeciesisabletomodulateitsdrinkingpostureinrelationtothewatersource;itcandrinkfromverythinfilmsoffreshwatereitherontheseasurfaceoronthesurfaceofintertidalmud,andcanevendrinkdirectlyfromfallingrainorfromwaterdropletsonvegetation(Davenport&Macedo,1990).Malaclemyscanalsoforciblyexpelwateroutofthebucco-pharyngealcavitywhendisturbedduringthedrinkingsequence,orwhenthevolumeofwaterwithinthiscavityistoogreat.Malaclemyscanexpelwaterveryquickly(lessthan1s)byasuddengapeincreaseaccompaniedbythroatelevationandneckretraction(Belsetal.,1995).
Asrecognizedforallvertebrates,feedingbehaviorinturtlesisamodal-action-pattern(MAD),involvingmovementsofthetrophicelementsintegratedwithneckdisplacements,sometimesasso-ciatedwithcomplexlimbmovements,sometimesnot.Allofthesecoordinatedsensori-motormove-mentsdefinethefeedingstrategyofturtles.Anyfooditemconsumedbyturtles(fromplantmaterialtofast-movingmobileprey)mustbelocatedandingestedbyarhythmicseriesofhead,jaw,andhyo-lingualmovements.Therearethreemajorsuccessivephasesinterrestrialturtles(Schwenk,2000):ingestion(capture),transportandintrabuccalmanipulation,andswallowing.Afourthphasecalledpharyngealpackingoccursattheendofseveralfeedingsequencesbutdoesnotinvolveanyjawopening-closingcyclicmovement(Figure8.1).Sincetheearliestfunctionalstudiesofvertebrates,acleardifferencehasbeendemonstratedbetweenfeedingstrategiesinwaterandonland(Liem,1990).Incompressiblewatercanbeusedtodriveamobilefooditem(livingprey)intothebuccalcavitybyproducingnegativepressureinsidethatcavity.Whenfoodiscaught,itmustbetransportedandswallowed.
Allfeedingphasesarebasedonrhythmiccoordinatedseriesofmovementsofthetrophicele-ments (e.g., jaws, tongue, hybranchium) associated with neck movements producing whole dis-placement of the head (Figure8.1). These movements are probably the result of rhythmic jawmovementsgeneratedbyacentralneuronalpopulationcalledthecentralpattern(orrhythm)gener-ator(CPG)(Schwenk,2000).Allrhythmicjawopening-closingactivitiesaremodulatedbysensoryfeedbackgeneratedbyvariousstructureswithin thebuccalcavity.Forexample, tastebudshavebeendescribedin thebuccalandtongueepitheliumofseveralspeciesof turtles(Iwasaki,1992,2002;Iwasakietal.,1996a,1996b,1996c,1996d;Beisseretal.,2001).Althoughalargenumberof
3339.indb 189 11/26/07 12:08:17 PM
��0 BiologyofTurtles
studiesprovideinformationontheforagingbehaviorofturtles,noquantitativeanalysishasactu-allydemonstratedtheeffectoffoodproperties(i.e.,texture,volume,size)onthemodulationofthemovementsofthetrophicsystem(e.g.,jaws,tongue,andhyobranchium).Inthemajorityofstudies,turtleshavebeenfedinthelaboratoryonstandardizedpreyitems(e.g.,livefishaselusiveprey)orplantmaterial(e.g.,fruitsandlettuceitemsforterrestrialspecies,availableplantsforaquaticspe-cies)forherbivorousspecies.
Foraquaticturtlesmainlylivingonthesubstratum,huntingstrategiesusuallyinvolveimmobil-itywhileawaitingpreyproximitybeforestriking,ormovingslowlytowardtheprey.Forexample,averyslow(0.4cms-1)stalkingmotionisreportedinthehighlycamouflagedpleurodiranChelus fimbriatusthatmovestowarditspreyuntilthetipofthesnoutisclosetothepreyitself(Lemellet al., 2002).Lemell andWeisgram (1997)described the feeding sequence in the aquaticPelu-sios castaneuswhilecatchingpreyonthesubstratumbyassociatingmovementsoftheturtleandthe use of the trophic system. Lauder and Prendergast (1992) described a preparatory phase in
figure�.� SeriesofframesdepictingthesuccessiveeventsofatypicalfeedingsequenceinGeochelone radiataeatingapieceofgrape.(A)Ingestioncycle,(B)transportcycle,(C)swallowingorpharyngealpack-ing,and(D)pharyngealcompression.Foodingestionalwaysinvolvescontactbythetonguepriortosurround-ingbythejawsforgrasping.Foodingestionandtransportinvolverhythmiccyclicjawandtonguemovements.Intransport,theforwardmovementofthetongueismodulatedbythepositionofthefoodinthebuccalcavity.Inswallowing,themouthisopenedandthefoodmovedtowardthepharynx.Inpharyngealcompression,thejawsalwaysremainclosed.Thetimingofeachframeisprovidedinms(lastthreenumbersrecordedontheframes).
3339.indb 190 11/26/07 12:08:19 PM
FunctionalEvolutionofFeedingBehaviorinTurtles ���
Chelydra serpentinafeedingonvariouspreytypesthatconsistsofaslow,voluntarystalkingmove-mentwithlittlehorizontalbodyandneckmovementbeforeafastopeningofthemouthassociatedwithasuddenneckextension(seeChapter7).SlowstalkingmovementpriortomouthopeningwasalsoreportedinM. terrapineatingfoodonthesubstratum(Belsetal.,1998).
Fromafunctionalpointofview,itisratherdifficulttodeterminetheintegrationbetweenloco-motorandtrophicsystemsfortheseturtlesbecauseinthelaboratory,foodisoftenpresentedattooshortadistancefromtheheadtomeasurethekinematicandhydrodynamiccharacteristicsofthefeedingbehavior.However,itisclearthattheneuromotorintegrationbetweenbothsystemsplaysaparticularlykeyrolewhenturtlesarehuntinginthewatercolumn,orwhenthepreyitemsshowspecializedbehaviororphysical texture (e.g., shells inmollusksandcrustaceans).Manyaquaticturtlesmustassociatecomplexlimbmovementswithneckmovementsandrhythmicmovementsofthetrophicsystemtosupportthecomplexmaneuveringabilitythatimprovesfoodcapturesuc-cess(Figure8.2).DavenportandClough(1985)reportanapparentlyuniquefeedingbehaviorthatinvolvedtheuseoftheforeflipperstogainfoodinthemarineturtleCaretta caretta.Theseauthorsobservedtheroleof“pseudoclaws”(sharp-pointedscales)ontheproximalportionsoftheforelimbsofyoungspecimenstoallowthecombineduseofforeflippersandbeaktotearfooditemsthatareotherwisetoolargetobeswallowedwholeorreadilybittenintochunks(thusmimickingthefeedingbehavioroffreshwaterturtlessuchasemydidsthatusetrueclawsandthebeaktoachieveasimilargoal)andallowingfoodmorselsadheringtothepseudoclawstobeingestedbytheturtle.
Feedingbehaviorinthebrackish-wateremydidM. terrapinisthebestexamplecurrentlyavail-able in the literature todemonstrateplasticity inneuromotor integrationof limb,neck,and tro-phicsystemsduringpreycapture(Belsetal.,1998).Capturingimmobileprey(e.g.,mussels)on
figure�.� (continued)
3339.indb 191 11/26/07 12:09:20 PM
��� BiologyofTurtles
thesubstratumismainlyachievedbycoordinatedneckextensionandjawopeningwithrelativelysmallinertialsuction(VanDamme&Aerts,1997;seefollowingfordiscussion).Capturingmobileandaggressivelydefensiveprey in thewatercolumn(e.g., livingcrabs) requirescoordinationofneckextensionandjawopeningwiththelimbcyclesusedforswimming.Inallcases,theneckisextendedtoplacetheopeningjawsclosetothepreyduringlimbretraction,toincreasethethrustoftheturtletowardtheprey(Figure8.2).Anothersortofcoordinationoflimb,neck,andjawshasalsobeendescribed.BelsandRenous(1992)andBelsetal.(1998)demonstratedthatfeedingonsoft,slowlyswimmingprey(jellyfish)byyoungleatherbackturtlesalwaysinvolvesplacementofthelongforeflippersalongsidethebodyduringthecatchingcycles.Theysuggestthatthisstrategystronglydecreasesthepossibilityoflimbmovementsdisplacingjellyfishawayfromthebuccalcav-ity.Interrestrialhabitats,turtlesoftenapproachfoodslowlyandstopallmovementsbeforecaptur-ingthepreyorbitingtheplantmaterial.
Theuseofthelimbsinallotherfeedingphasesremainsrelativelypoorlyunderstood.Forturtlesfeedinginthewatercolumn(e.g.,Emydidae),thelimbsplayamajorruleinoptimizingfoodtrans-port(Belsetal.,1998);typically,theforelimbclawsareusedtotearlargefooditemsintosmallerpieces.However,forturtlesthatfeedinterrestrialhabitats,thelimbsonlyplayanoccasionalrolebystabilizingthepositionofafooditemonthesubstratumoraidingtheseizureof“difficult”hardfooditemsbetweenthejaws(e.g.,Testudinidaeeatinglivingpreysuchassnails).Alloftheseexamplesshowthatfunctionalstudiesmustbeecologicallyrelevant toyieldafullunderstandingof turtlefeedingstrategiesandtheirplasticitythatplaysakeyroleintheoverallfitnessoftheorganism.
�.� KinematiCs
8.5.1 terreStrIAlfeedIng
�.�.�.� ingestion
Twomodesof foodprehensionhavebeenreportedfor terrestrial turtles: lingualprehensionandjawprehension(Figure8.1&Figure8.3).AcleardifferenceiningestioncycleshasbeenreportedforTerrapenesp.feedingoninsectsandGeochelonesp.feedingonplantmaterial.T. carolinaandT. ornataneveruselingualprehensionbutonlyjawprehensionwhencatchinginsects(e.g.,Belsetal.,1997).Geochelonesp.andTestudosp.alwaysuselingualprehensionforfeedingonplantmaterial.Formobileprey (mealworms, asused inTerrapene studies),Kinixys alsouses lingualprehension.However,noquantitativecomparativeanalysesofthemodulatoryeffectsoffooditems
figure�.� TwotypicalfeedingsequencesofMalaclemysattackingadefendingcrab.Thesesequencesshowthecomplexinteractionbetweentheturtleandthelivingprey.However,imagesshowthestereotypedpatternofneckextension,gapecycle,andthroatdepressionforbothattemptstoingestpartsofthecrab.Thearrowshowsthejumpingbehaviorofthecrabattackingtheturtlethatdefendsbybiting(correspondingtoingestioncycle).
3339.indb 192 11/26/07 12:09:21 PM
FunctionalEvolutionofFeedingBehaviorinTurtles ���
onkinematicandmotorvariableshaveyetbeenreportedintheserepresentativetortoisesofTes-tudinidae.Incasesoflivingprey(e.g.,insects),onlyoneingestioncycleisusedtocatchthefoodanddrivethefooditemintothefrontofthebuccalcavity.InTerrapenesp.studiedwiththisfooditem,thejawmovesquicklyaroundthepreyandthetongueisneverusedforprehensionofinsects(Belsetal.,1997).Forplantmaterial,theingestion(capture)cycleismorecomplexanddependsonthesizeofthefooditembecausethefeedingboutisacontinuousprocessthatdoesnotterminateatoneparticularphase.Twoexamplesdemonstratethiscomplexity.Forrelativelylargefooditemssuchasgrapes,G. radiatacontactsthefoodwiththetongueandretractsthefooditemwithinthebuccalcavitywhilethejawsaremovingaroundthefood.Theturtleusuallyusesonetothreeinges-tioncyclestobringthefoodmaterialsuccessfullyintoitsbuccalcavity.SimilarbehaviorhasbeenreportedfortheherbivorouslizardUromastixfedonendive(Herreletal.,2002).TheG. radiatahead is thenplaced in an approximatelyhorizontal positionduring the immediately subsequentcycles,allowingtransportandintrabuccalmanipulation;for“long”fooditemssuchaslettuceleavesthataredrawnintothemouthbyrhythmicjawcycles,thefooditemsarecaughtbythetongueandthejawsmovethepieceoffoodaroundseveraltimesbeforethefoodpartlyorcompletelyentersthebuccalcavity.Itisratherdifficulttoseparatefoodingestionandtransportbecauseateachtonguecycle(Figure8.1andFigure8.3),thefoodwithinthebuccalcavityismovedbythemidandposte-riorportionsofthetonguewhiletheforetonguemovestoadheretothepartsofthefooditemthatarestilloutsideofthebuccalcavity.Whenapieceoffoodiscutoff,theturtlecanstoptoingesttherestofthefooditem,whichcontinuestobeactedupononlybytransportcycles.Suchacontinuous
figure�.� SeriesofframesdepictingthesuccessiveeventsofatypicalfeedingsequenceinKinixys bel-lianaeatingapieceoflettuce:(A)ingestioncycleand(B)transportcycle.Foodprehensiondoesnotinvolvecontactbythetongueoutsidethebuccalcavity.Foodtransportinvolvesrhythmiccyclicjawandtonguemove-ment.Contactbetweentongueandfoodoccurswithinthebuccalcavity.Intransport,theforwardmovementofthetonguethatplaysthekeyroleismodulatedbythepositionofthefoodinthebuccalcavity.Thetimingofeachframeisprovidedinms(lastthreenumbersrecordedontheframes).
3339.indb 193 11/26/07 12:09:23 PM
��� BiologyofTurtles
processofingestion-transportisstronglyinfluencedbythesizeofthefoodmaterial.Whenashortpieceoflettuceisprovidedtoturtles,onlyoneortwoingestioncyclesareusedtodealwiththefood.Theeffectofthesizeofthefooditemonthecontinuousprocessinvolvingtheforetonguetocatchthefooditemandthemid-andhindtongueportionsfortransportingthefoodhasbeenclearlydem-onstratedinallherbivorousmammalsfeedingonlongpiecesofhay(Bels,2006),sothisprocessisnotunusualinvertebrates.AssoonasthefoodiswithinthebuccalcavityofG. radiata,aseriesofintegratedhead(neck),jaw,andhyolingualcyclestermedtransport-intrabuccalmanipulationmovethefoodposteriorlytowardthepharynx.Attheendofacompletesequence,cyclesdeterminingtheswallowingphasetypicallyshowadifferentgapeprofileashasbeennotedinherbivorouslizards(Herrel&DeVree,1999).
Inbothmodesoffoodprehension,turtlesalwaysusecombinedmovementofthejaws,thehead,andtheneckthatmayormaynotbeassociatedwithdisplacementsofthehyolingualapparatus.Allingestioncyclesinallterrestrialturtlesareaccompaniedbyneckextension,movingtheopeningjawstowardthefooditem(Figure8.4).Duringthenextextension,theheadispositionedrelativetothefooditemdependingonthepropertiesofthefoodanditsproximalcues(i.e.,size,volume,texture,mobility),showingacomplexsensory-motormechanismcontrollingtheinitiationofthisfeedingphase.Ateachingestioncycle,theturtlerotatestheheadinvariouspositionstobeabletotakeholdoftheplantmaterial.Thesensory-motormechanismsunderlyingsuchfunctionalplastic-ityduringingestionremainunderstudied.Interrestrialturtles,thequestionofpositioningtheheadtogetherwithaneckextensionremainsanopenquestion.Probablydifferencesinthespeedofheadmovementstowardthefoodarekeyparametersthatpermitthesereptilestomodulateheadrotationduringfoodingestion.Thisdifferencecanbeseenasafunctionalconsequenceofdietselectioninterrestrialturtles.
Twomaintypesofingestioncycleshavebeenreported:jawprehensionandlingualprehension.Inbothmodesoffoodprehension,turtlesalwaysusecombinedmovementsofthejaws,thehead,andtheneck,associatedornotwithdisplacementsofthehyolingualapparatus.Inallprehension,themouthisopenedinslow(SO)andfast(FO)stages(Figure8.4).However,thedurationofSOstageisvariable.InT. carolinaandT. ornata,acleardistinctionbetweenSOandFOstagewasnotalwayseasytodeterminebecausethegapeincreasesratherregularly,andoftenthemeandurationofFOstageislongerthanthatofthefastclosing(FC)stage.ThetonguealwaysmovesforwardwithinthebuccalcavityduringgapeincreasesandretractsduringthebeginningoftheFOstage.Uponprotrusion,thetonguebulgesandthetonguetipcurvesventrally,sothattheforetonguemakescontactwiththefooditem.DuringtheSOphase,thetongueisprotrudedfromthemouthandcon-tactsthefood.Next,thetonguecontinuestobeextendedandpushedagainstthefooditem.Thefoodisthenmaintainedincontactwiththetonguebecauseofstrongmucousadhesionbetweenthetonguesurfaceandthefood.Oncethetonguehasbeenretractedwithinthejawmargins,thejawsareclosedquickly,andtheslowclosing/powerstrokephasestarted.ThemeandurationoftheFOstageislongerthanthatoftheFCstage.Duringthedecreaseingapesize,thelowerjawarrivesunderthebeakformedbytheupperjawsasintransportcycles.Thetonguemovesforwardwhilethegapeincreasesandcontactsthefoodbeforemaximalgapeisachieved.RetractionofthetongueoccursduringthebeginningoftheFOstage.Afterclosingthejawsontheprey,theheadretractsand rotates in reverse.TimingofkinematiceventsofG. elephantopus (lingualprehension)andT. carolina(jawprehension)issimilarinthesedifferentspecies(Figure8.4).Forexample,peakhyoidretractionoccurs justafterpeakgapeangleandmaximumthroatdepressionoccurs30 to100msaftermaximumgapeangle.Thesuccessivesixeventsinbothturtlescanbesummarizedasfollows:
NeckextensiontodrivetheheadtowardthefoodOpeningofthemouth(SOandFOstages)RetractionofthetongueduringthebeginningoftheFOstageDepressionofthethroatimmediatelyfollowingthetongueretraction
••••
3339.indb 194 11/26/07 12:09:23 PM
FunctionalEvolutionofFeedingBehaviorinTurtles ���
ClosingofthemouthonthefoodRetractionoftheheadtowardthecarapace.
�.�.�.� intra-oraltransportCycle
Assoonasthefood(e.g.,livingprey,plantmaterial)entersthebuccalcavity,transportofthefoodtowardthepharynxbegins.Forexample,afteringestionofamealworm,thepreyisnotcompletelyheldwithin thebuccalcavityand is reduced(i.e.,partiallycrushed)by the jawmarginsateachtransportcycle(Figure8.3andFigure8.5).Becausethemealwormentersthebuccalcavitypro-gressively,successiveportionsofthemealwormarereducedattheclosingstageofeachtransportcycle.
TheslowopeningofthemouthduringtransportiscomposedoftheSOI(opening)andoftenSOII(stationarystage)stages.TheFOstagecorrespondstoarapidincreaseintheverticaldisplace-mentofthejaws(Figure8.5).TheFOstagebeginsafteraslightdecreaseingapeangleattheend
••
50A
B
SO FO
–1 1 2 3Time (s)TTOR
Maximal Gape
TBNE
TMNE
TTOR
TBTD
TMTD
–1.0 –0.5 0.0 0.5 1.0Time (s)
G. elephantopus C. carolinaTime (s)
–1.0 –0.5 0.0 0.5 1.0
Maximal Gape
0–2
FC SC
25
0
Mea
n G
ape A
ngle
(d
egre
es)
figure�.� (A)Meangapeprofile (±standarddeviation) from10 lateral ingestioncycles inonespeci-menofGeocheloneelephantopus.Thearrowindicatesthemeantimeoftongueretraction.(B)ComparisonbetweentimingeventsinG.elephantopusandT. carolina(datafromBelsetal.,1997).Timingswerecalcu-latedfromthetimetopeakgapeangle(time0).Gapeisdividedintoslowopening(SO),fastopening(FO),fastclosing(FC),andslowclosing(SC).TBNE:timetobeginningoftheneckextension,TMNE:timetomaximalneckextensionduringingestioncycle,TTOR:timetotongueretraction,TBTD:timetobeginningofthroatdepression,TMTD:timetomaximumthroatdepression.
3339.indb 195 11/26/07 12:09:25 PM
��� BiologyofTurtles
ofSOstage.Theclosureofthemouthisdividedintofastclosing(FC)andslowclosing(SC)stages.TheFCstagecorrespondstoarapiddecreaseintheverticaldisplacementofthejaws.TheSCstagecorrespondstotheelevationofthelowerjawunderthebeakoftheupperjaw.Thehorizontaldis-tancebetweenthetipoftheupperjawandlowerjawdecreasesduringtheFCstagebecauseverticaldisplacementofthelowerjawproducesanarc,andtheupperjawmoveslittle.TheSCstageisnotreflectedinrecordablegapeangleandgapeverticaldistancechangesbecausethelowerjawmovesundertheedgesoftheupperjaw.However,duringthisstagethehorizontaldistancebetweenthetipoftheupperjawandthemostanteriorpointofthelowerjawincreasesjustafterFCstageinthegapecycle,indicatingthatthelowerjawcontinuestocloseagainsttheupperjaw.WhengapeincreasesduringtheSOstageofthenextcycle,thishorizontaldistancedecreasesagain.
Inthetransportcycle,duringtheSOstagethetongueisprotractedasillustratedbyadecreaseofthehorizontaldistancebetweenthetipsofthetongueandthelowerjaw(Figure8.3BandFig-ure8.5).Atthesametime,thetongueisslightlyelevated.Inthetransportcycle,theforwarddis-placementofthetongueintothebuccalcavityisstronglycorrelatedwiththeverticaldisplacementofthelowerjawduringtheSOstage.Whenthetonguemovesforward, thelowerjawdepresses
10
5
Gap
e Dist
ance
(cm
)
Jaw
Vert
ical
Mov
emen
ts(c
m)
Thro
atVe
rtic
alM
ovem
ent
(cm
)
Hea
dM
ovem
ent T
owar
dth
e Foo
d (c
m)
0
TH
LJ
UJGA
EY
Upper jaw
Lower jaw
10
5
0
5
0
00 1 2 3 4 5 6
Time (s)7 8 9 10 11 12
20
10
figure�.� KinematicprofilesoftwosuccessiveintraoralfoodtransportcyclesinG. elephantopus.Throatverticalmovement(TH)associatedwithtongueretractionshowsthecyclicdepressionandelevationofthethroat.Headmovementtowardthefoodwasmeasuredbythehorizontaldisplacementoftheeye(EY).Thismovementshowsthecyclicdisplacementofthejawstowardthefoodassociatedwithgapeangle(GA)pro-ducedbycombinationofverticalmovementoftheupper(UJ)andlower(LJ)jaws.Thearrowsindicatethefirstframeoftongueretractionwiththefoodadheringtothetonguesurfaceandthelineindicatespeakgapeangleforeachofthegapecycles.
3339.indb 196 11/26/07 12:09:26 PM
FunctionalEvolutionofFeedingBehaviorinTurtles ���
(SOI),andwhenthetongueisstationarypriortomovingbackwardduringtheFOstage,thelowerjawdoesnotmoveandthegapeangleremainsstationary(SOII).TheheadextendsduringtheFOstageandthebeginningoftheFCstage,anddepressesduringtheSO,FO,andthebeginningofFCstages.DuringhalfofthedurationoftheSOstage,theheadrotatesdownwardasshowedbytheverticaldisplacementofthehead.Preliminaryanalysisoffoodtransportbyusingx-rayvideo(250Hz)inatypicalplantfeedingturtle,K. belliana,showsthatthefoodisaccumulatedwithinthepharynxandtherearpartof thebuccalcavity(Figure8.6).Subsequently,swallowingofthealimentarybolusbegins.
�.�.�.� swallowing
AccordingtoSchwenk(2000),wecandivideswallowinginterrestrialturtlesintopharyngealpack-ing(openingofmouth)andpharyngealcompression(nogapecycle).Figure8.3andFigure8.6showtypicalswallowingcycle in twoT. carolina (Figure8.3)andK. belliana (Figure8.6)feedingondifferentfooditems(mealwormsinT. carolinaandlettuceleavesinK. belliana).Probablybasedonthesensory-motorfeedbacksfrompropertiesofthealimentarybolus(i.e.,volume,size,tough-ness),theturtleeitherentersintoanewfeedingsequencethatdrivesmorefoodintothepharynx,
figure�.� (A)TypicalingestioncycleofapieceoflettuceinK. bellianafilmedbyx-rayvideofluoroscopy(100Hz),showingthetonguecontactingthefoodwhereasthejawsmovedtosurroundthefoodmaterial(leafoflettuce).Thecontactbetweenthetongueandthefoodoccurswithinthebuccalcavityattime0.(B)Typicalswallowingcyclefilmedbyx-rayfluoroscopy(200Hz)involvingmouthopeningtoshowthecombinedmove-mentofthefoodassociatedwiththeswallowingcycle,demonstratingthekeyroleofthetongueandthroatintheposteriordisplacementofthefoodtowardtheesophagus.Peristalticactivityoftheesophagushelpingmovementofthefoodwithintheesophagusisalsoshown.Thedisplacementofthefoodalongpharyngealpackingisshownbythearrowoneachframe.Time0correspondsforthiscycletothefirstframeofmouthopening.f:markers(thelettucewascoveredwithathinfilmofwaterchargedwithbariumpowder).
3339.indb 197 11/26/07 12:09:27 PM
��� BiologyofTurtles
ormovesthebolusintotheesophagus,whichstructuredrivesthebolusdirectlytothestomachbyperistalticmovement.Foremptyingthepharynx,theturtlesproduceeitherjawcyclesorpharyngealcompression(Figure8.6).
8.5.2 AquAtIcfeedIng
Feeding in aquatic turtles has received more attention than that of terrestrial turtles, althoughrelativelyfewspecieshavebeenstudied.However,robustexperimentalconditionswithmore-or-lesssimilarenvironmentalconstraintshavebeenemployed.SincethefirstquantitativeanalysisoffeedingmechanisminChelydra(Lauder&Prendergast,1992),turtleshavebeenusedfortestinghypothesesandgeneralizationsregardingmorphologicalandfunctionalpatternsassociatedwithaquaticfeedinginlowervertebrates.
Thelargenumberofspeciesandforagingstrategiesinaquatic-feedingturtlesprobablyreflectsaratheruniquegroupofvertebratesthathasevolvedaquaticfeedingconvergentlywithanamniotefeedingsystemsandwithamniotesthathavereturnedsecondarilytoaquaticenvironmentsuchasseveralmammals.Manyfoodresourcescanbeexploitedinaquatichabitats.Plantmaterialconsti-tutesafirstsourceoffood.Thismaterialcanbefirmlyattachedtothesubstrateandmustbebittenandextractedorpulledoutbeforebeingtransportedandswallowed.Otherplantmaterialcanbefloatingandmustbebittenandcaughtfrombeneath(Davenportetal.,1992).Livingpreycanbeexploitedbyrapidstrikesiftheyhaveelusiveabilities(e.g.,fishes)ormustbeapproachedslowlytoavoidtheirmovementawayofthebuccalcavity(e.g.,jellyfish).Sincetheearliestanalysesoffeed-ingmechanismsinwater,thequestionofimportanceofsuctionhasremainedparamount(Aertsetal.,2001;Figure8.7).
�.�.�.� ingestionCycle
Aquaticturtlesallshowmuchthesamepatternofhead,jaws,andhyobranchiummovements.Toourknowledge,thetongueisnotusedforcapturingaquaticprey.Typicalcapturecyclesforsomeaquaticturtles(C. longicollis,C. fimbriatus,C. serpentina)involveasuddenforwardthrustoftheheadbyneckextension(seeChapter7).Incontrast,otherspeciesstrikelessrapidly(meandurationbetween250and300msinM. terrapin,Belsetal.,1998;400msinT. carolina,Summersetal.,1998;andupto800msinD. coriacea,thischapter),openingthemoutheitherbyusingSOandFOstagesorregularlywithouttheSOstage.Allkinematicprofilesavailableintheliteratureprovideclassicalexamplesofneckextensionproducingasuddenthrustoftheopeningmouthassociatedwithlargedepressionofthehyobranchium.Depressionofthehyobranchiumoccurs30to50msaftermaximumgape(Lauder&Prendergast,1992;Bels&Renous,1992;VanDamme&Aerts,1997;Belsetal.,1998;Summersetal.,1998;Lemelletal.,2002).
Theproblemofapproaching the food inaquaticenvironmentalwaysremains thesame:anyflow of water produced by the turtle must be compensated for in efficient capture. Lauder andPrendergast (1992)werethefirst tostress thehydrodynamicconstraintsonaquaticpreycaptureresultinginkinematicsimilaritiesamongpredatorssuchasfish,amphibians,andturtles.Theirfind-ingsweresupportedbyotheranalysesofingestioninturtlesfromfreshwaterandmarinehabitats.Alloftheaquaticturtlescangenerateabackwardwaterflowrelativetothebuccalcavity(calledsuction)toacquirethefood(VanDamme&Aerts,1997).VanDammeandAerts(1997)discussedindepththequestionofcompensatoryandinertialsuctioninpreycapturebyaquaticturtles.Theterm“compensatorysuction”indicatessuctionusedtomaintainfoodimmobileduringthestrike,andpermitsengulfingofthefoodasthejawapparatussurroundsit.Theterm“inertialsuction”isusedtodescribesuctioninwhichfoodandwatermovetowardthebuccalcavitywhereastheheadofthepredatorremainsessentiallyimmobile(VanDamme&Aerts,1997;Aertsetal.,2001).Aertsetal.(2001)statethatprobablyfoodcaptureinvariousaquaticturtlesisrelatedtoacombinationof both modes of suction, as demonstrated by displacements of various food items recorded indifferentaquaticturtles.Forexample,inertialsuctionplaysamajorroleinChelodina longicollis
3339.indb 198 11/26/07 12:09:27 PM
FunctionalEvolutionofFeedingBehaviorinTurtles ���
butcompensatorysuctionisdominantinC. serpentina(VanDamme&Aerts,1997).VanDammeandAerts(1997)andSummersetal.(1998)donotagreewiththeuseoftheram/suctionindex(RSI)proposedbyNortonandBrainerd (1993) todescribe the feedingmechanismsoffishes. In theirrecentstudy,Lemelletal.(2002)appliedthisindextocaptureofbyC. fimbriatus.TheequationforRSIis
CBI
10–4 m2
10–2 m
10–2 m
6
A B
C
D
Frame 692
Frame 726
Frame 742
Frame 750
Frame 756
Frame 766
Frame 792
Frame 822
4
2
0
2
1
1
2
0
0
0 50 100Milliseconds
756
750 766 792 922692 742726
150
–1
Are
a of C
ross
-sec
tion
Disp
lace
men
tD
ispla
cem
ent
CBII
Gape
Head
Carapace
PREYebfr
PREYtbfr
Neck
??
figure�.� (A) Drawings of lateral and ventral views of the strike used for illustrating the kinematicprofiles.(B–D)KinematicprofilesofonerepresentativestrikeinChelodina longicollistoshow(B)themodi-ficationofareasofcrosssectionsthroughthreesuccessivelevelsontheheadoftheturtle(VanDamme&Aerts,1997):themouth(gape)andatthelevelofceratobranchialI(CBI)andII(CBII),thedisplacement,oftheprey,thehead,andthecarapaceoftheturtleintheearthboundframeofreference(ebfr=fixedframeofreference).Timescaleisgiveninmsandnumbersoftheuppertimescalecorrespondtothenumberofthesequencespresentedattheleftofthefigure.(ModifiedfromVanDamme&Aerts,1997.)
3339.indb 199 11/26/07 12:09:31 PM
�00 BiologyofTurtles
RS I =
D - D
D + Dpredator prey
predator prey
where Dpredator and Dprey are the net distances moved by the predator and the prey between themomentthemouthfirstbeginstoopenandthemomentthepreydisappearsorisseizedbythejaws).RSIrangesfrom+1,indicatingapureramstrikeinwhichonlythepredatormoves,to−1,indicatingapuresuctionstrikeinwhichonlythepreymoves.ThecalculatedRSIduringprey(fish)captureinP. castaneusvariesbetween0.36and0.55,andinC. fimbriatuswasalwayspositive(mean=0.36±0.23,range=0.071to0.664;prey=fish;N=20).However,theseauthorsthinkthattheequationusedforRSItendstooverestimatetheramcomponent.
Whatever the terms used for describing food capture (e.g., suction, RSI), and whatever thehydrodynamic calculationsor other considerations involved, all aquatic turtlesmust expand theoropharyngealcavityforsuccessfulfoodcapture.Summersetal.(1998)demonstrateclearlythatpreycaptureinwaterinvolvesgreaterhyoiddepressioninT. carolinafeedinginwatercomparedwithfeedingonland.TheseauthorsandBelsetal.(1998)showthathyoiddepressionismodulatednotonlyfromlandtowaterbutalsoinwaterfeedinginrelationtofoodtypeandbehavior(e.g.,crabscapableofdefensivebehavior).Itisevidentthatthisexpansionplaysakeyroleinthesuc-cessfulcaptureofelusivepreybyanymodeofpreydisplacementtowardthebuccalcavity(inertialsuction)orofmaintainingthepreyimmobileinthewatercolumn(compensatorysuction).Aertsetal.(2001)suggestthatfunctionaldemandsrelatedtofeedinginwateralwaysrelatetoexpansionoftheareaposteriortothejawapparatusthatopenstoallowpreyandwatertoenterpartlyorcom-pletelyintotheoropharyngealcavity.However,theroleofthisexpansionbecomeslessimportantinturtlesfeedinguponplantmaterialfloatingatthesurface,pullingmaterialoutofthesubstratum,orattackingavigorouslydefendingprey.Inthelattercase,bitingperformancebythejawsprobablyplaysthekeyrolebecauseitallowstheturtletoobtainfoodandalsodefendagainsttheattackoftheself-protectingpotentialprey(Herreletal.,2002).Despitelargestructuralvariations,theexpansionoftheoropharyngealcavityismainlyproducedbymovementofthehyobranchiumasnicelydem-onstratedbyx-rayfilms(Aertsetal.,2001;Lemelletal.,2002).ForillustratingthemotorcontrolofthroatexpansioninChelonida,fewdataareavailable.Aertsetal.(2001)providedthefirstdescrip-tionofmotorsequencesthatshowdepressionandretractionofthehyoidbody(Figure8.8).Lemelletal.(2002)reportthattheesophagusisfilledwiththelargeamountofwatersuckedinduringthegapecycleuntilthemouthisclosed.Theseauthorsprovideacompletex-rayanalysisofpreymove-mentwithinthebucco-pharyngealcavity(Figure8.9).Inallturtles,thepreyiseithersuckedinward(inertialsuction)orbittenbyclosingjaws(compensatorysuction).Inthemeantime,thethroatislaterallyexpandedbyrotationof lateralelements (ceratobranchials I)of thehyobranchium.Thedisplacementof theelementsof thehyoidapparatushasalwaysbeenassumedtoproducethroatexpansioninallstudiedaquaticturtles(Lemell&Weisgram,1997;VanDamme&Aerts,1997;Lemelletal.,2002),andnowthereisclearconfirmatoryevidence.Theposteriorpartofthethroatisexpandedbyrotationoftheposteriorlateralelementsofthehyobranchium(cetarobranchialsII;VanDamme&Aerts,1997;Lemelletal.,2002).Themouthisthenagainopenedslightlytoexpeltheexcesswaterbyreturningthehyoidapparatustoitsstartingposition,andthepreyisretainedonthefloorofthebuccalcavity.
Allmajordataonfoodingestioninaquaticturtleshavebeencollectedinenvironmentalcondi-tions(e.g.,temperature,salinity)closetotheclassicalrelevantecologicalconditionsdeterminedforthestudiedspecies.Forexample,Belsetal.(1998)recordedfeedingbehaviorinM. terrapinat25°Cinwaterwithsalinityof33psu.Leatherbackturtleswerefilmedat26°Cinclassicalartificialsea-water(Bels&Renous,1992).However,performancesinturtles(asinallreptiles)canbemodulatedinrelationshiptotemperature.Itwouldbeexpectedinectothermicturtlesthatspeedsofjawactionwouldincreasewithhighertemperatureswithinthephysiologicalrangeofaspecies,andviceversa.
3339.indb 200 11/26/07 12:09:32 PM
FunctionalEvolutionofFeedingBehaviorinTurtles �0�
Giventhedifferentthermalconditionsoftheirnaturalranges,wecomparedgapeperformancesofthreeaquaticspecies(Figure8.10).Inthisstudy,itwasnecessarytostudyjawactionatarangeofrealistic temperatures foreachspecies.Datawereobtained forTrachemys (Emydidae)at18,24and30°C.Cuora(Emydidae)andSiebenrockiella(Bataguridae)wouldnotfeedat18°C;theywerefilmedat24,28,and30°C(threespecimensofeachspecieswereheldovernightformorethan14hoursateachstudytemperaturebeforebeingfilmed).Figure8.3andFigure8.4respectivelyshowtheeffectsoftemperatureonbitetime(i.e.,timetocontactfood)andjawopeningtime(i.e.,timetomaximumgape)foradultsofallthreespecies.ItisevidentthatthetwoemydidspecieswerebothlessaffectedbytemperaturechangesthanSiebenrockiella,whichshowedasteepdeclineinbitetimeandjawopeningtimebetween24and30°C(at24and30°C,one-wayANOVAcomparisonsamongthethreespeciesrevealedhighlysignificantdifferences,p<0.005).Trachemysshowednochangeinbitetimeandjawopeningbetween24and30°C;evenat18°Ctherewasrelativelylittleslowingofjawactionincomparisonwith30°C(bitetimeby24%,jawopeningtimeby55%).TukeyposthoctestsshowedthatTrachemyswassignificantlyfaster(shorterbite/jawopeningtimes)thanCuoraatcommontemperatures(24and30°C;p<0.05inallcases)andalsosignificantlyfaster(p<0.05)thanSiebenrockiellaat24butnotat30°C.Dataforbitetimeweremorevariablethanforjawopeningtime;thispossiblyreflectsvariationinfoodmorselsizeandthepositioningofthejawsrelativetofoodduringjawclosure.Jawopeningtimeappearstobemoreusefulforcomparisonsandissolelypresentedfortherestoftheresults.Differencesbetweenthetwoemydidspecieswereparticularlymarked,withjawopeningtimebeing227%longerinCuorathaninTrachemysat24°C,and200%longerat30°C;clearly,TrachemysbitesfarfasterthanitsAsianrelative.
These resultsprovide thefirst relevant functionaldata toexplain the roleof feedingperfor-mancesinthesuccessofinvasionofTrachymysturtlesthroughouttheaquatichabitatsoftheworld.
cm2
8 CB1 CB1
A B
CB1
Lower jaw
Hypoglossum
Hyoid
mDM
mIC
mIC
mCH
mBM
mBM
mCH
mBH
mBH
CB2 CB2
CB2 Configuration
Adducted Expanded
Gape
mDM
mBM
mBH
mCH
mIC
Onset exp.
Max. gape
Mouth closed
50 ms
4 0
figure�.� (A) Schematic representation of the head skeleton of C. longicollis in the adducted andexpandedconfigurations.(B)ResultsofelectromyographicalanalysisduringexpansionofthehyobranchiuminC. longicollis(fromAertsetal.,2001).ThegraphrepresentsthechangeofthecrosssectionalsurfaceoftheoropharyngealcavityatthelevelofthegapeandthroughthethroatatthelevelofceratobranchialsIandII.Arrowsindicatepositionandorientationof themajorexpansionmuscles.CB1:ceratobranchialI;CB2:ceratobranchialII;mBH:m.branchiohyoideus;mBM:m.branchiomandibularis;mCH:m.coracohyoideus;mDM:m.depressormandibulae;mIC:m.intercornuatus.,exp.:throatexpansion(Aertsetal.,2001).
3339.indb 201 11/26/07 12:09:33 PM
�0� BiologyofTurtles
Wedemonstrate too that the invasiveTrachemys bites faster over awide rangeof temperaturesrelativetothetwoAsianspecies(Cuora,Siebenrockiella)whosehabitatitnowshares.Thissug-geststhatitcandealmoreeffectivelywithelusiveordangerouspreyaswellasfeedingoncarrionorvegetationmorequickly.ThethermaldataalsoindicatethatTrachemysiscapableofeffectivefeedingoverawidertemperaturerangethanthetwonativespecies,allowingittocontinuefeed-ingundercoolerconditions.Theintroductionofanomnivorous,highlyeffectivecompetitortoanecosystemisclearlyundesirable,butthesituationismadeworsebytheunusualcharacteristicsof
figure�.� Successiveframesofahigh-speedx-rayfilmsequence(150frames/s)showingalateralviewofC. fimbriatusduringfoodcapture.Thetime(s:ms)isprovidedforeachframe.Thepreyitemappearsdarkbecauseofthex-raycontrastmedium.Leadmarkerswerealsopositionedontheskulloftheturtle.(FromLemelletal.,2002.)
3339.indb 202 11/26/07 12:09:34 PM
FunctionalEvolutionofFeedingBehaviorinTurtles �0�
Trachemysintroductions.Mostinvasivespeciesdonotbecomeestablishedinnewhabitatsbecausetheyfailtoreproduce,ordonothaveimmunitytopathogenspresentintheirnewhabitat.WhereasferalTrachemyshavebeenshowntobreedinJapan,Israel,Germany,andFrance(Ernstetal.,1994;Cadietal.,2004),thegreatbulkoftheirnumbersinAsia(aselsewhere)stemfromrepeatedandcontinuinglarge-scaleintroductions.Tosomeextent,theirabilitytobreed/notbreedisirrelevant,particularlyas theyareverylonglived,oftensurviving30+years inthewild(Kuhrt&Dewey,2002).AccordingtotheTurtleConservationFund(2002),Asiais“thegeographicregionthatwar-rantsthehighestpriorityifwearetoavoidlosing[chelonian]speciesinthenearfuture.”Theresultsofourcomparativebiomechanicalstudyprovidefurtherindicationofthenecessityfortrappingandremovingthishighlycompetitiveintroducedturtlefromnon-nativeecosystems.
0
70 Gape angle
L food
CU
A
C
B
60 Trachemys
Trachemys
Siebenrockiella
Siebenrockiella
Cuora
Cuora
SiebenrockiellaTrachemys
Cuora
50 40 30
Gap
e Ang
le (°
)
20 10
0
700
600
500
400
300
Tim
e to
Bite
Foo
d (m
s)
200
100
0 100 200 300
Time (ms) 400 500 600 15 20 25
Temperature ()
Temperature (°C)
35 30
600
500
400
300
Jaw
Ope
ning
Tim
e (m
s)
200
100
0 15 20 25 35 30
figure�.�0 (A)Examplesofgapeanglesequencesinthethreestudyspeciesat30°C.Thebodymassesofthethreespecimensfilmedwereasfollows:Trachemys(492g),Siebenrockiella(701g),andCuora(1041g).(B)Effectoftemperatureonbitetimes(timetocontactwithfood)inadultTrachemys,Cuora,andSieberocki-ella.Symbolsrepresentmeanvalues(n=3)andstandarddeviations.(C)Effectoftemperatureonjawopen-ingtimes(timetomaximumgape)inadultTrachemys,Cuora,andSieberockiella.Symbolsrepresentmeanvalues(n=3)andstandarddeviations.
3339.indb 203 11/26/07 12:09:36 PM
�0� BiologyofTurtles
�.�.�.� manipulationandtransportCycle
Although poorly analyzed, transport of thefood is more complex because it involves twomaintypesofuseofbothelementsofthehyo-branchium: the tongue and the hyoid appara-tus.InM. terrapin,thetongueplaysakeyrolein manipulating food in association with thedepression of the hyobranchium (Figure8.11).For transporting the food, the tongue is alsoclearly associated with classical depression ofthe hyobranchium in helping backward dis-placementofthefoodwithintheoropharyngealcavity.Inotherspecies,thefoodcanbesuckedinwithout any actionof the tongue.Basedondifferences in movement of the hyobranchiuminC. fimbriatus,Lemelletal.(2002)describedtwo modes of transport cycles involving slowsuction effects on the prey within the buccalcavity(Figure8.12).Inthefirstmode,thehyoidis depressed slightly and the mouth is openenoughtofacilitatethereleaseofthepreyfromthejaws,withthefishbeingheldattheendoftheceratobranchials.Inthesecondmode,hyoiddepressionisofthesameextentasduringpreycapture.Thevolumeoftheanteriorpartoftheesophagusincreasesslowlyandthepreyisheldbetweentheceratobranchials.Theturtleexpelsthewater very slowly and theprey remains attheendofthehyoidapparatus.
8.5.3 feedIngInDermochelys coriacea:AtypIcAlexAmpleofAmArIneturtlewIthAhIghlySpecIAlIzeddIet
�.�.�.� materialsandmethods
Fouryoungleatherbackturtles(80to500g)havebeenfilmedat200to300Hzusing16mmfilms.TheturtlesfromFrenchGuianawereincubatedat30.5°Candkeptinanaquariumof2.3to5.0m3at25°Cinseawater(pH=8.0to8.1;salinity=32g/l).Theanimalswerefilmedunder500Wwhenfedwithcrudemussels(Belsetal.,1988).Thefoodwaspresentedgentlyinfrontoftheturtles.Onlytruelateralsequenceswerekeptfortheanalysisusingthetypicalmethoddetailedpreviously(Belsetal.,1998)andpointsweredigitizedtocomparemovementsofthejaws,thehyobranchium,andthelimbsduringthesuccessivephasesofthefeedingbehavior(Figure8.12andFigure8.13).
8.5.3.2reSultS
FeedingboutsinD. coriaceaconsistofsuccessivejawandhyolingualcyclesfrompreycapturetoitstransportintotheesophagus.Duringthefeedingbouts,theturtlesstabilizethebodybyslowdisplacementsoftheforelimbsthatarenotrelatedtothesuccessivegapeopenings.Twotypesofjawcycleswereobserved: ingestion (includingbitingof the smoothmaterial that is cut at eachcycle)andtransportcycles.Wedidnotrecordanyspecificswallowingcycleswiththetypeofsoft
figure�.�� Typical stages of food (entire mus-sel)manipulationinM. terrapin.Thetongueplaysamajorroleinmovingfoodfromonesideofthejawsto the other. The food is partially crushed at eachclosingofthejaws.Timebetweenframes:0.08s.
3339.indb 204 11/26/07 12:09:37 PM
FunctionalEvolutionofFeedingBehaviorinTurtles �0�
foodusedinthisstudy(piecesofmusselflesh).Insuccessiveingestioncycles,thefoodentersthe
buccalcavityandisreducedbetweentheclosingjaws.However,insomeoftheseingestioncycles,
transportalsooccurswhenthefoodismaintainedinfrontoftheturtles.Intruetransportcycles
(Schwenk,2000),thefoodistakencompletelyintothebuccalcavityandthenmovesposteriorlyto
theesophaguswithoutanyreductionbetweenthejaws.However,thefoodmaybereducedbetween
thehardpalateand the tongue.Suchreductionandbitingcyclesproducesmallparticulatemat-
terthatisejectedfromthemouthduringtheslowopeningofthenextjawcycle.Throatelevation
duringthetimebetweenthetwoFOstagesofsuccessivecyclesproducedmovementofwater(and
particulatematter)outofthebuccalcavity(Figure8.13).
figure�.�� Successiveframesofahigh-speedx-rayfilmsequence(150frames/s)showingalateralviewofC. fimbriatusduringfoodtransport.Thetime(s:ms)isprovidedforeachframe.Thepreyitemappearsdarkbecauseofthex-raycontrastmedium.Leadmarkerswerealsopositionedontheskulloftheturtle(figurefromLemelletal.,2002).
3339.indb 205 11/26/07 12:09:38 PM
�0� BiologyofTurtles
Kinematically,eachgapecycleofthebitingandtransportphasesinvolvesaslowopening(SO),fastopening(FO),andclosingstage(C).TheSOstageofbothcycle types ishighlyvariable indurationandamplitude.Insomecycles,itinvolvesa“stationarystage”(SOII)priortothesuddenincreaseoftheFOstage;inothers,itdoesnot.TheSOstagemaybealsocompletelyabsentinsomecycles.Fromhigh-speedcinematographicfilms, itwasnotpossible to recordclear relationshipsbetween thepresenceanddurationof thisSOstage,with thecycle functionor the tongue-foodposition.ThemeandurationofSOstageinbitingcyclewassignificantlylongerthanofSOstageinthetransportcycle(Kruskall-WallisANOVA,T=4.7;df=18;P<0.05),whereasFOandCstageswerenotsignificantlydifferent.
Themouthisopenedbyacombinationofventraldepressionofthelowerjawanddorsaleleva-tionoftheupperjaw.Typically,thehyoid-tonguedisplacementsarenotdifferentiningestionandtransportcycles.DuringtheSOstageandthefirsthalfoftheFOstage,thehyoid-tonguecomplex
A
PPTO
FO
SO 2/22B
SO 1/16
9/22
GAGA
FC
7/16
4/16
TO
FO
11/22
C20/22
12/16
figure�.�� Successiveframesshowing(A)ingestionand(B)transportcyclesinD. coriacea(high-speed16-mmfilmsat100Hz).Thegapeisdividedinslowopening(SO),fastopening(FO),andclosing(C)stages—thedivisioninfastclosing(FC)andslowclosing(SC)stageswasnotalwaysclearduringtheingestioncycle.Inertialsuctionplaysakeyroleinbothfeedingphases.Thedurationoftheingestioncycleis0.22s(22frames)andthedurationofthetransportcycleis0.16s(16frames).Thenumbersindicatedateachdrawingcorrespondtothenumberofframesrelativetothetotalnumberofthecycle.TimetopeakgapeisindicatedbyGA.Con-tactbetweenthefoodandthetonguewasalwaysobservedandtongueretractswiththefoodasthethroatisdepressed.TO:tongue.
3339.indb 206 11/26/07 12:09:43 PM
FunctionalEvolutionofFeedingBehaviorinTurtles �0�
iselevatedandprotractedasillustratedbydecreasingofthethroatangle.ThedistancebetweenthemandibleandthehyoidbodyvisiblethroughthethroatskindecreasesstronglyduringSOstageandis0.07±0.01spriortomaximalgapeamplitude(endofFOstage).Thethroatexpansionthenoccursduring0.14±0.05sattheendofFOandFCstages.IncasesofastationarystagepriortoaSOstageorveryslowopeningstage, thehyoid-tonguedistancedoesnotchangegreatlypriordecreasing.Therewasnosignificantdifferencebetweendisplacementsofthehyoidpointonthethroatduringbitingandtransportcycles(Figure8.14).
100Ingestion
Upper jaw
Lower jaw
Transport-swallowing
50
80
40
0
5
0
5
0
5
00 1 2
Time (s)3
ca neFood
4
Gap
e (de
gree
s)
Jaw
Ver
tical
Mov
emen
ts(c
m)
Thro
at d
istan
ce I
(cm
)Th
roat
Dist
ance
II(c
m)
Nec
k H
oriz
onta
lM
ovem
ent
(cm
)
0
figure�.�� KinematicprofilesofingestionandtransportcyclesinD. coriaceafeedingonsoftmaterial(piecesofmussels)withpropertiesclose to thematerialcaught in thenaturalenvironment (jellyfish).Wecategorizeingestioncyclesasallcyclesusedformovingfoodintothebuccalcavity,andtransportcyclesasallcyclesthattakeplacewithfoodinsidethebuccalcavity.Gapecyclesinbothphasesaredividedintoslowopening(SO),fastopening(FO),andfastclosing(FC).Divisionbetweenclosinginfastclosing(FC)andslowclosing(SC)isnotyetclear.However,attheendoftheclosingstagetheangularaccelerationofthegapeangledecreasesslightly.Thekinematicprofilesillustratingthethroatdisplacementfollowasimilarpatternduringbitingandtransportcycles.ThethroatdistancesIandIIwerecalculatedasthedistancecorrespondingtothelinetracedfromonepointonthethroattothehorizontallinepassingthroughtheedgeofthemandibleateachframeatanangleof90°.ThethroatdistanceIcorrespondstothehyoidbodyandthethroatdistanceIItotheregionoftheceratobranchialsI.DuringtheSOandfirsthalfoftheFOstagesofeachcycle,thethroatdis-tanceIiselevated0.01sbeforethethroatdistanceII(posterior).Retractionofthehyoid-tonguesystembeginsduringtheFOstageandoccursduringtheclosingstage.Peakhyoidretractionbeginswithorjustbefore(0.01s)fastclosing.Thehyoidapparatusismaximallyretracteduntilthefastclosing(FC)isachieved.TheneckdistancewascalculatedasthedistancebetweenpointNE(endoftheskull)andCA(carapace).
3339.indb 207 11/26/07 12:09:44 PM
�0� BiologyofTurtles
DuringSOandFOstages,watermovesintothebuccalcavitysimultaneouslyasthepreythatmoveseithertothebuccalcavity(biting)orposteriorlyintothebuccalcavity(transport).Anaddi-tionalinertialsuctionmechanismhelpsdisplacepreytowardthepharyngealcavity;thisismainlyproducedbytongueretractioninbitingandtransportcycles.Strongcontactsbetweenpreysurfaceandtonguedorsalsurfacewereobservedinbothcycles.
�.� evolutionoffeedingBehaviorinturtles
Discussionofevolutionoffeedingbehaviorinturtlesremainscontroversial.BasedontheirstudyofpreycaptureinT. carolina,Summersetal.(1998)statethat“thecurrentlyacceptedhypothesisisthattheancestortorecentturtleswasterrestrial(Gaffneyetal.,1987),butthatanaquaticlifestyleevolvedearlyinthehistoryofthecladeandisprobablyprimitiveforallextantturtles(Gaffneyetal.,1987,1991;Carroll,1988).”Theseauthorsconcludedthatterrestrialfeedinginturtlesisaderivedbehavior(Belsetal.,1997;Summersetal.,1998).Hereweprovidesomediscussionthathighlightstheneedforfuturequantitativekinematicandmotoranalysesinterrestrialandaquaticturtles.
8.6.1 IngeStIon
Terrestrialfeedingmodesincludelingualandjawprehension.Bothmodesareusedforvariousfooditems.ExceptinTerrapene(Belsetal.,1997;Summersetal.,1998;Bels,2003),lingualprehensionisthemainmodeforallstudiedturtlesoftheTestudinidae.Thefoodadherestothetongueeitherin(e.g.,Kinixys)orout(e.g.,Geochelone)ofthebuccalcavity.Summersetal.(1998)suggestthatT. carolinausesaterrestrialfeedingmodeinwaterbecausethevelocitiesofallskullelementsduringfoodcapturebygeneralistsareslowerthanthoseofspecialists.Fromavailabledata,threehypoth-esescanbesuggested.First,theingestioncyclehasbeenderivedinthe“true”terrestrialcheloni-ans(i.e.,Testudinidae)showingatongue-basedintraoralfeedingbehaviorfromfoodtransportandreductiontoswallowing.Ingestioncanbeviewedasanevolutionarytransformationofatongue-basedfeedingmechanismforefficientprocurementoffood.Thecoordinationofthetonguemove-menthasnotchangedgreatly,withthetonguemovingslightlytowardthefoodduringjawopeningandretractingthefoodaftercontact;alternatively,thejawapparatuscansimplysurroundthefooditemandthetonguemakescontactwiththefoodwithinthebuccalcavitypriortobeingretracted.Second,bothmodesofprehension(lingualandjawprehensions)werepresentinancestorsofturtlesandcanbeusedinvariouswaysdeterminedbythepropertiesofthefood.Suchmodulationinfoodprehensionhasbeenreportedforsquamates(Schwenk,2000).Third,terrestrialturtlesderivedfromaquaticancestorshave“reinvented”thekeyroleofthetongueforimprovingefficiencyoffoodpro-curementtogetherwiththeirconquestofterrestrialhabitats.Inthiscase,wemustadmitthatlingualprehensionisaderivedpatternconstrainedbythepropertiesofthefoodresourcesbecomingavail-ableduringtheevolutionofterrestrialturtles.InTerrapene,whichbelongstoaprimitivelyaquaticclade,terrestrialfeedingisasecondarilyderivedmodeinwhichthejawprehensionthatplaysakeyroleinaquaticfeedinghasbeenconserved.Summersetal.(1998)suggestthatT. carolinawouldusetongueprehensiononpreythatislargerthanmealworms.WehavenotyetconfirmedthissuggestionbyexaminingprehensionofvarioustypesoffoodinTerrapene(Bels,personalobservation).
8.6.2 trAnSport(AndotherfeedIngphASeS)
Theevolutionoffeedingbehaviorandmotorcontrolofturtlesstillremainsdifficulttofullyunder-stand.First,theoriginofturtlescontinuestobelargelyproblematic.Second,onlyafewspecieshavebeenextensivelystudied,mainlyintermsoffeedingmechanismsandnotfromanevolution-aryperspective.However,availabledatacanprovidesomeinsight,particularlywithrespecttothelikelihoodofsomepossiblehypothesesconcerningtheevolutionoffeedingbehaviorinchelonians.
3339.indb 208 11/26/07 12:09:45 PM
FunctionalEvolutionofFeedingBehaviorinTurtles �0�
Acomparativeanalysisofallfeedingphases—exceptfoodcapture—appearsinteresting.LauderandGillis(1997)comparedtransportkinematicsandmechanismsoftrulyaquaticvertebrates(e.g.,fishesandamphibians),firstwiththoseofamphibiansthatfeedinbothterrestrialandaquatichabi-tats,andsecondwiththoseoftrulyterrestrialAmniota(e.g.,squamates).Theysuggestthatseveraltraitsrecordedforsquamatesarenovelfeaturesofthefeedingmechanismthatappearedwiththeconquest of terrestrial habitats and can therefore be considered as plesiomorphic for tetrapods.Thesetraits includediversityofintraoralprocessing,thepresenceofaSOstagepriortosuddengapeincrease(FOstage),andhyoidandtongueprotractionduringSOproducedbyauniquepatternofhyoidmusclecontraction(Lauder&Gillis,1997).Thesesuggestionsagreewiththeevolution-aryapproach to feedingbehaviorproposedbyReillyandLauder (1990),whoadvocatea singlegeneralizedmodeloffoodtransportintetrapods.Morerecently,McBrayerandReilly(2002)donotsupportallthefeaturesofBrambleandWake’s(1985)generalmodel(i.e.,presenceofSOIandSOIIinslowopening).Thesetwomodelshavebeenproposedtodescribethegeneralizedgapepatternandtonguemovementduringfoodtransportinterrestrialvertebrates.Basedonavailabledatain1985,BrambleandWake(1985)presentedahypotheticalgeneralizedmodelforancestraltetrapodswithgapebeingdividedintoslowopening(SO),fastopening(FO),fastclosing(FC),andslowclos-ing(SC-PS)stages.BeforeFO,thegapeincreaseisdividedintoslowopeningI(SOI)involving“acomparativelylowgapeangle”(Bramble&Wake,1985)whilethetongueslidesbeneaththefoodandaslowopeningII(SOII),that“isrecognizedbyadistinctdeclineintherateofchangeofgape”(Bramble&Wake,1985).Thislatterstageisrepresentedinthemodelbyaplateauinthegapecycle.ThenduringFO,thefoodismovedposteriorlybyactionofthetongue.AfterFO,themouthclosesrapidly(FC)andthenmoreslowlyasthejawscontactthepreyduringSC-PSstages.
Reilly and Lauder (1990) proposed another generalized model for amniotes. These authorsdividetheincreasingstageofthegapecycle(openingofthemouth)onlyintoslowopening(SO)andfastopening(FO),withnoSOIIstage.Thisdifferenceingapeincreaseishighlysignificantbecauseitemphasizesadifferenceintonguemovementduringtransportingofthefood—seeMcBrayer&Reilly(2002)foradiscussionbasedonquantitativedatainalargesetofsquamates.AccordingtoLee(1997),thedevelopmentoftheshellandratherflattenedbodyofturtlescouldbelinkedtoanancestralherbivorousdiet.Itmaybeassumedthatancestralformswereterrestrialandfedonplantmaterial(andperhapssmall,slowlymovingpreyorcarrion),asisthecaseforalargenumberofextantspeciesstudiedtodate(e.g.,Geochelonesp.,Kinixyssp.).Basedonthishypothesis,wemayconcludethatavailabledatasupporttheconclusionthatthecharacteristicsoftransportcyclesareplesiomorphicforterrestrialfeedinginturtles,althoughthereisalargevarietyindietandspecial-izationofthehyobranchium,yieldingthepresenceofaSOstagebeforeFOofthemouth,amove-mentofthetongueunderthefoodduringtheSO,andretractionofthefoodbythetongueatSO-FOtransitionoratthebeginningofSOstage.
Intheirquantitativeanalysis,Belsetal.(1997)comparedtheirdatainterrestrialfeedingbyT.carolinawiththetwopreviouslydescribedgeneralizedmodelsfortetrapods.Availabledatadonotpermitcategoricalconclusions.However,arapidsurveyofkinematicsfacilitatesthecomparisonofaquaticandterrestrialtransportphases.Inallturtles,withalltypesoffood,thejawcycleisoftendividedintoSO,FO,andclosingstages.TheSOstageisalwayspresentinallterrestrialturtles.Thisstagehasalsobeenreportedinalargenumberofaquaticturtles(P.castaneus,Lemell&Weisgram,1997;T. carolina,Belsetal.,1997;Summersetal.,1998;M.terrapin,Belsetal.,1998;D. coria-cea,Belsetal.,1998;C. frimbriatus,Lemelletal.,2002).Wesuggestthatthereisanintra-oraltransportcyclethatissimilarforallterrestrialturtles.Itisalsoevidentthatthetongueisusedinalargenumberofaquaticturtlestotransportfooditems,justasinterrestrialturtles.ItsprotractionoccursduringtheSOstageanditretractswiththefoodattheboundarybetweenSOandFOstagesorearlyduringFOstage.Wemaycallthismechanismintra-oral aquatic lingual transport.Incon-trast,suctionplaysthedominantrolefortransportingfoodinsomespeciessuchasC. fimbriatus(Lemelletal.,2002).Wemaycallthismechanismintra-oral aquatic hyoid transport.Probably,therelativesizeofthetongueisthelimitingmorphologicalfactorindetermininguseoftongue-based
3339.indb 209 11/26/07 12:09:45 PM
��0 BiologyofTurtles
intra-oraltransportorhyoid-basedintra-oraltransport.Theslowlyopeningmouthcangeneratelowpressurewithinthemouthcavitybecauseofprotractionofthehyoidduringthisstageinbothcases.However,thisisrapidlycompensatedforbysuctionthatoccursduringthroatexpansionaftermaxi-mumgape.DuringtheFOstages,rapidmouthopeningisimmediatelyfollowedbyaposteroventralmovementofthehyoidapparatus;highnegativepressureoccurswithintheoropharyngealcavity,whichisproducedbypeakhyoiddepressionfollowingpeakgapeinallstudiedturtles.DuringtheFCphase,thehyoidapparatusremainsdepresseduntilthemouthisclosed.
aCKnoWledgments
Theauthorsthankallthosewhohelpedtogatherthedatausedinthispaper.TheyareverygratefultoS.Attereforherhelpinwritingthispaper,andtopersonsfordiscussionandhelpinvariouspartsofthiswork(G.Daghfous,S.Montuelle).Wethanktwoanonymousreviewersfortheirhelpfulcomments.
referenCes
Aerts,P.,VanDamme,J.,andHerrel,A.,Intrinsicmechanicsandcontroloffastcraniocervicalmovementsinaquaticfeedingturtles,Am. Zool.,41,1299,2001.
Beisser,C.J.,Lemell,P.,andWeisgram,J.,LightandelectronmicroscopyofthedorsallingualepitheliumofPelusioscastaneus(Pleurodira,Chelidae)withspecialrespecttoitsfeedingmechanics,Tiss. Cell,33,63,2001.
Bels,V.L.,EvaluatingthecomplexityofthetrophicsysteminReptilia, inBiomechanics and Evolution of Vertebrates,V.L.Bels,J.-P.Gasc,andA.Casinos(eds.),Oxford:BiosPublishers,2003,185.
Bels,V.L.,Feeding in Domestic Vertebrates: From Structure to Behaviour,Wallingford,CT:CabiPublish-ing,2006.
Bels,V.L.,Davenport,J.,andDelheusy,V.,KinematicanalysisofthefeedingbehaviorintheboxturtleTer-rapene carolina(L.),(Reptilia:Emydidae),J. Exp. Zool.,277,198,1997.
Bels,V.L.,Davenport,J.,andRenous,S.,FoodingestionintheestuarineturtleMalaclemys terrapin:Com-parisonwithmarineleatherbackturtleDermochelys coriacea,J. Mar. Biol. Assoc. UK,78,953,1998.
Bels,V.L.,Davenport,J.,andRenous,S.,DrinkingandwaterexpulsioninthediamondbackterrapinMalacle-mys terrapin (Latreille).J.Zool.,236:483–497,1995.
Bels,V.L.,andRenous,S.,Kinematicsoffeedingintwomarineturtles(Chelonia mydasandDermochelys coriacea),inProceedings of the Sixth Ordinary General Meeting S.E.H.,Z.KorsosandI.Kiss(eds.),Budapest:HungarianNaturalHistoryMuseumPress,1992,73.
Bels,V.L.,Rimblot-Baly,F.,andLescure,J.,CroissanceetmaintienencaptivitédelatortueLuth,Dermo-chelys coriacea(Vandelli,1761),Rev. Fr. Aquariol.,15,59,1988.
Bouchard, S.S., and Bjorndal, K.A., Nonadditive interactions between animal and plant diet items in anomnivorousfreshwaterturtleTrachemys scripta,Comp. Biochem. Phys. B,144,77,2006.
Bramble, D.M., and Wake, D.B., Feeding mechanisms of lower tetrapods, in Functional Vertebrate Mor-phology,M.Hildebrand,D.M.Bramble,K.F.Liem,andD.B.Wake(eds.),Cambridge,MA:HarvardUniversityPress,1985,230.
Cadi,A.,Delmas,V.,Prévot-Julliard,A.-C.,Joly,P.,Pieau,C.,andGirondot,M.,Successfulreproductionoftheintroducedsliderturtle(Trachemys scripta elegans)intheSouthofFrance,Aquat. Conserv.,14,237–246,2004.
Carroll,R.L.,Vertebrate Paleontology and Evolution,NewYork:W.H.Freeman&Co,1988.Cebra-Thomas,J.,Tan,F.,Sistla,S.,Estes,E.,Bender,G.,Kim,C.,Riccio,P.,andGilbert,S.F.,Howtheturtle
formsitsshell:Aparacrinehypothesisofcarapaceformation,J. Exp. Zool. B,304,558,2005.Claude,J.,Pritchard,P.,Tong,H.,Paradis,E.,andAuffray,J.-C.,Ecologicalcorrelatesandevolutionarydiver-
genceintheskullofturtles:Ageometricmorphometricassessment,Syst. Biol.,53,937,2004.Cleuren,J.,andDeVree,F.,KinematicsofthejawandhyolingualapparatusduringfeedinginCaiman croco-
dilus,J. Morphol.,212,141,1992.Davenport,J.,andClough,W.,Theuseoflimbscalesor“pseudoclaws“infoodhandlingbyyoungloggerhead
turtles,Copeia,1985,786,1985.Davenport,J.,andClough,W.,Swimminganddivinginyoungloggerheadseaturtles(Caretta carettaL.),
Copeia,1986,53,1986.
3339.indb 210 11/26/07 12:09:46 PM
FunctionalEvolutionofFeedingBehaviorinTurtles ���
Davenport,J.,andMacedo,E.,BehaviouralosmoticcontrolintheeuryhalinediamondbackterrapinMalacle-mys terrapinLatreille:Responsestolowsalinityandrainfall,J. Zool.,220,487,1990.
Davenport,J.,Spikes,M.,Thornton,S.M.,andKelly,O.B.,Crab-eatinginthediamondbackterrapinMalacle-mys terrapin:Dealingwithdangerousprey,J. Mar. Biol. Assoc. UK,72,835,1992.
Davenport,J.,Wong,T.M.,andEast,J.,FeedinganddigestionintheomnivorousestuarineturtleBatagur baska (Gray),Herpetol. J.,2,133,1992.
Ernst,C.H.,andBarbour,R.W.,Turtles of the World,Washington,DC:SmithsonianInstitutionPress,1989.Ernst,C.H.,Lovich, J.E., andBarbour,R.W.,Turtles of the United States and Canada,Washington,DC:
SmithsonianInstitutionPress,1994.Gaffney,E.S.,Aphylogenyandclassificationofthehighercategoriesofturtles,Bull. Am. Mus. Nat. Hist.,
155,387,1975.Gaffney,E.S.,Comparativecranialmorphologyofrecentandfossilturtles,Bull. Am. Mus. Natur. Hist.,164,
67,1979.Gaffney,E.S.,Hutchinson,J.H.,Jenkins,F.A.,andMeeker,L.J.,Modernturtleorigins:Theoldestknown
cryptodire,Science,237,289,1987.Gaffney,E.S.,Meylan,P.A.,andWyss,A.R.,Acomputerassistedanalysisoftherelationshipsofthehigher
categoriesofturtles,Cladistics,7,313,1991.Harless,M.,andMorlock,H.,Turtles: Perspectives and Research,NewYork:JohnWiley&Sons,1979.Herrel,A.,andDeVree,F.,KinematicsofintraoraltransportandswallowingintheherbivorouslizardUro-
mastixacanthinurus,J. Exp. Biol.,202,1127,1999.Herrel,A.,O’Reilly,J.C.,andRichmond,A.M.,Evolutionofbiteperformanceinturtles,J. Evol. Biol.,15,
1083,2002.Hill,R.V.,IntegrationofmorphologicaldatasetsforphylogeneticanalysisofAmniota:Theimportanceof
integumentarycharactersandincreasedtaxonomicsampling,Syst. Biol.,54,530,2005.Hughes,G.R.,Luschi,P.,Mencacci,R.,andPapi,F.,The7000-kmoceanicjourneyofaleatherbackturtle
trackedbysatellite,J. Exp. Mar. Biol. Ecol.,229,209,1998.Iwasaki,S.,Finestructureofthedorsalepitheliumofthetongueofthefreshwaterturtle,Geoclemys reevesii
(Chelonia,Emydinae),J. Morph.,211,125,1992.Iwasaki,S.,Evolutionofthestructureandfunctionofthevertebratetongue,J. Anat.,201,1,2002.Iwasaki,S.,Asami,T.,andWanichanon,C.,Finestructureofthedorsallingualepitheliumofthejuvenile
hawksbillturtle,Eretmochelys imbricatabissa,Anat. Rec.,244,437,1996a.Iwasaki,S.,Asami,T.,andWanichanon,C.,Ultrastructuralstudyofthedorsallingualepitheliumofthesoft-
shellturtle,Trionix cartilaginous(Chelonia,Trionychidae),Anat. Rec.,246,305,1996b.Iwasaki,S.,Wanichanon,C.,andAsami,T.,Histologicalandultrastructuralstudyofthelingualepitheliumofthe
juvenilePacificridleyturtle,Lepidochelys olivacea(Chelonia,Chelonidae),Ann. Anat.,178,243,1996c.Iwasaki,S.,Wanichanon,C.,andAsami,T.,UltrastructuralstudyofthedorsallingualepitheliumoftheAsian
snail-eatingturtle,Malayemys subtrijuga,Ann. Anat.,178,145,1996d.Joyce,W.G.,andGauthier,J.A.,PalaeoecologyofTriassicstemturtlesshedsnewlightonturtleorigins,Proc.
R.S. B.-Biol. Sci.,271,1,2004.Kear,B.P.,andLee,M.S.,AprimitiveprotostegidfromAustraliaandearlyseaturtleevolution,Biol. Lett.,
2,116,2006.Kuhrt,T.,andDewey,T.,“Trachemysscripta“,AnimalDiversityWeb,http://animaldiversity.ummz.umich.
edu/site/accounts/information/Trachemys_scripta.html,2002.Lauder,G.V.,andGillis,G.B.,Originoftheamniotefeedingmechanism:Experimentalanalysesofoutgroup
clades,inAmniote Origins: Completing the Transition to Land,S.SumidaandK.Martin(eds.),SanDiego,CA:AcademicPress,1997,152.
Lauder,G.V.,andPrendergast,T.,KinematicsofaquaticpreycaptureinthesnappingturtleChelydra serpen-tina,J. Exp.Biol.,164,55,1992.
Laurin,M.,andReisz,R.R.,Areevaluationofearlyamniotephylogeny,Zool. J. Linn. Soc.,113,165,1995.Lee,M.S.Y.,Correlatedprogressionandtheoriginofturtles,Nature,379,812,1997.Lemell,P.,Beisser,C.J.,andWeisgram,J.,FeedingpatternsofPelusios castaneus(Chelonia:Pleurodira)with
commentsonthemorphologyofitstongue,J. Morphol.,232,285,1997.LemellP.,Lemell,C.,Snelderwaard,P.,Gumpenberger,M.,Wocheslander,R.,andWeisgram,J.,Feeding
patternsofChelus fimbriatus(Pleurodira:Chelidae),J. Exp. Zool.,205,1495,2002.Lemell,P., andWeisgram, J.,The feedingpatternsofPelusios castaneus (Chelonia:Pleurodira),Neth. J.
Zool.,47,429,1997.
3339.indb 211 11/26/07 12:09:46 PM
��� BiologyofTurtles
Liem,K.F.,Aquaticversusterrestrialfeedingmodes:Possibleimpactsonthetrophicecologyofvertebrates,Am. Zool.,30,209,1990.
Lindeman,P.V.,Evolutionoftherelativewidthoftheheadandalveolarsurfacesinmapturtles(Testudines:EmydidaeGraptemys),Biol. J. Linn. Soc.,69,549,2000.
McBrayer,L.D.,andReilly,S.M.,Testingamniotemodelsofpreytransportkinematics:Aquantitativeanaly-sisofmouthopeningpatternsinlizards,Zoology,105,71,2002.
McCauley,S.J.,andBjorndal,K.A.,Responsetodietarydilutioninanomnivorousfreshwaterturtle:Implica-tionsforontogeneticdietaryshifts,Physiol. Biochem. Zool.,72,101,1999.
Nagashima,H.,Uchida,K.,Yamamoto,K.,Kuraka,S.,Usuda,R.,andKuratani,S.,Turtle-chickenchimera:Anexperimentalapproachtounderstandingevolutionaryinnovationintheturtle,Dev. Dyn.,232,149,2005.
Norton,S.F.,andBrainerd,E.L.,Convergenceinthefeedingmechanicsofecomorphologicallysimilarspe-ciesintheCentrarchidaeandCichlidae,J. Exp. Biol.,176,11,1993.
Pough,F.H.,Andrews,R.M.,Cadle,J.E.,Crump,M.L.,Savitzky,A.H.,andWells,K.D.,Herpetology,UpperSaddleRiver,NJ:Prentice-Hall,2001.
Pritchard,P.C.H.,Encyclopedia of Turtles,Neptune,NJ:T.F.H.Publications,1979.Reilly,S.M.,andLauder,G.V.,Theevolutionof tetrapodfeedingbehavior:Kinematichomologies inprey
transport,Evolution,44,1542,1990.Reina,R.D.,Jones,T.T.,andSpotila,J.R.,SaltandwaterregulationbytheleatherbackseaturtleDermochelys
coriacea,J.Exp. Biol.,205,1853,2002.Reisz,R.R.,andLaurin,M.,Owenettaandtheoriginofturtles,Nature,349,324,1991.Renous,S.,andBels,V.,EtudecinématiquedelapalettenatatoireantérieuredelatortueluthDermochelys
coriacea(Vandelli,1761)aucoursdesalocomotionterrestre,Can. J. Zool.,69,495,1989.Schwenk,K.,Feeding: Form, Function and Evolution in Tetrapod Vertebrates,SanDiego,CA:Academic
Press,2000.Shaffer,B.H.,Meylan,P.,andMcKnight,M.L.,Testsofturtlephylogeny:Molecular,morphologicalandpale-
ontologicalapproaches,Syst. Biol.,46,235,1997.Shedlock,A.M.,Botka,C.W.,Zhao,S.,Shetty,J.,Zhang,T.,Liu,J.S.,Deschavanne,P.J.,andEdwards,S.V.,
Phylogenomics of nonavian reptiles and the structure of the ancestral amniote genome, Proc. Natl. Acad. Sci. USA,104,2767,2007.
Smith,T.,Kardong,K.,andBels,V.,Preycapturebehaviorintheblue-tonguedskink,Tiliqua scincoides,J. Herpetol.,33,362,2000.
Summers,A.P.,Darouian,K.F.,Richmond,A.M.,andBrainerd,E.L.,KinematicsofaquaticandterrestrialpreycaptureinTerrapene carolina,withimplicationsfortheevolutionoffeedingincryptodireturtles,J. Exp. Zool.,281,280,1998.
VanDamme,J.,andAerts,P.,KinematicsandfunctionalmorphologyofaquaticfeedinginAustraliansideneckedturtles(Pleurodira:Chelodina),J. Morphol.,233,113,1997.
Wyneken,J.,Seaturtlelocomotion:mechanisms,behaviorandenergetics,inThe Biology of Sea Turtles,P.L.LutzandJ.A.Musick(eds.),BocaRaton,FL:CRCPress,1997,165.
3339.indb 212 11/26/07 12:09:46 PM