6.5a Strong and Weak Acids and Bases
-
Upload
ericka-galang -
Category
Documents
-
view
231 -
download
0
Transcript of 6.5a Strong and Weak Acids and Bases
-
8/13/2019 6.5a Strong and Weak Acids and Bases
1/14
Section 6.5 pg. 254-259
-
8/13/2019 6.5a Strong and Weak Acids and Bases
2/14
ACIDS BASES
Tastes sour
Turn blue litmus red
pH less than 7
Neutralize bases
React w/ active metals to
produce H2(g)
React w/ carbonates to
produce carbon dioxide
Taste bitter
Feel slippery
Turn red litmus blue
pH greater than 7
Neutralize acids
Fill in as many empirical properties for acids and bases as you can ...
-
8/13/2019 6.5a Strong and Weak Acids and Bases
3/14
Investigation 6.3:
Comparing the Properties of Acids (pg. 254)
Read over the Design. What are two importantcontrolled variables?
-
8/13/2019 6.5a Strong and Weak Acids and Bases
4/14
The evidence from the demonstration clearly shows that acids withthe same initial concentration can have different degrees of acidic
properties.
This difference was shown in the different conductivity
measurements and the different rate of reactions.
What were the two important controlled variables?
concentrationand temperature
Why? A very dilute strong acid could have a higher pH than a more
concentrated weak acid which is incorrect and the conductivity and
rate of reaction measurements would be misleading.
-
8/13/2019 6.5a Strong and Weak Acids and Bases
5/14
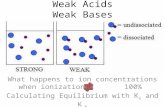
The concept of strong and weak acids was developed todescribe and explain the differences in properties of acids.
An acid can be described as a weak acid if itscharacteristic properties (under the same conditions) areless than those of a common strong acid
There are relatively few strong acids:
HClO4(aq), HI(aq), HBr(aq), HCl(aq)H2SO4(aq), HNO3(aq)
-
8/13/2019 6.5a Strong and Weak Acids and Bases
6/14
Strong Acids: have high conductivity, high rate of reactionw/ metals and carbonates and a relatively low pH
These empirical properties suggest many ions are present(lots of H3O
+ions present); which is consistent with the ideathat strong acids react completely (>99%) with water to
form hydronium ions
HCl(aq) + H2O(l) H3O+
(aq) + Cl-(aq)
>99%
-
8/13/2019 6.5a Strong and Weak Acids and Bases
7/14
Weak Acids: have low conductivity, a lower rate of reactionw/ active metals and carbonates and a relatively high pH
These empirical properties suggest fewer hydronium ionsare present
Based on this evidence, a weak acid reacts incompletely(
-
8/13/2019 6.5a Strong and Weak Acids and Bases
8/14
Because of this difference, weak acids are much safer tohandle you can even eat and drink many of them.
Lactic acidin many
dairyproductsand builds
up inmuscles
Citrus acid in fruits
Stearic Acid in Animal Fat
Carbonicacid indrinks
Tannic Acid in Tea
-
8/13/2019 6.5a Strong and Weak Acids and Bases
9/14
Pg. 255 #1-5
-
8/13/2019 6.5a Strong and Weak Acids and Bases
10/14
Strong bases
have a high electricalconductivity, fast reaction rate and a very highpH (>>7)
Weak bases have a low electrical conductivity(if molecular bases), slower reaction rate and a
pH closer to, but greater than, 7
How do we explain the difference?
-
8/13/2019 6.5a Strong and Weak Acids and Bases
11/14
Strong Bases
all soluble ionic hydroxides thatdissociate completely (>99%) to releasehydroxide ions
NaOH(s)
Na+
(aq) + OH-
(aq)
Weak Bases an ionic or molecular substancethat reacts partially (
-
8/13/2019 6.5a Strong and Weak Acids and Bases
12/14
Explain the weak base properties of baking soda.
NaHCO3 (aq)Na+
(aq) + HCO3-(aq)
HCO3-(aq) + H2O(aq)H2CO3(aq) + OH-(aq)
Explain why sodium acetate has a pH of 8 whendissolved in water.
NaCH3COO(aq) Na+
(aq) + CH3COO-(aq)
CH3COO-(aq)+ H2O(l) CH3COOH(aq) + OH
-(aq)
-
8/13/2019 6.5a Strong and Weak Acids and Bases
13/14
Pg. 257 #6, 7, 9
-
8/13/2019 6.5a Strong and Weak Acids and Bases
14/14
Strong Acids Weak Acids Strong Bases Weak Bases
Empiricalproperties
(need sameconcentration& temperature)
Very low pH Med to low pH Very high pH Med to high pH
High
conductivity
Low
conductivity
High
conductivity
Low
conductivity*
Fast reactionrate
Slow reactionrate
Fast reactionrate
Slow reactionrate
ModifiedArrhenius
Theory
Completelyreact with
water to formH3O
+(aq) ions
Partially reactwith water to
form H3O+(aq)ions
Completelyreact with
water to formOH-(aq) ions
Partially reactwith water to
form OH-(aq)ions
* Applies only to weak bases that are molecular