Lecture 5 Sept 9, 2005 MACROMOLECULES #1 Carbohydrates And Lipids.
5 macromolecules #1 Carbs & Lipids
Transcript of 5 macromolecules #1 Carbs & Lipids

Lecture 5 Lecture 5 Sept 9, 2005Sept 9, 2005MACROMOLECULES #1MACROMOLECULES #1
Carbohydrates Carbohydrates
And LipidsAnd Lipids
-- Polymers Polymers
--CarbohydratesCarbohydratesmonomers and polymersmonomers and polymers
-- LipidsLipids
Lecture outline:Lecture outline:
Principles of Building Polymers:Principles of Building Polymers:
-- biological polymers are built from biological polymers are built from simple small unitssimple small unitscalled called monomersmonomers
-- addition of each addition of each monomericmonomeric unit occurs with theunit occurs with theremoval of a removal of a water moleculewater molecule
A A condensation dehydrationcondensation dehydration reactionreaction
-- ends are chemically distinct ends are chemically distinct directionalitydirectionality of synthesis of synthesis
-- requires requires energy inputenergy input for polymerization;for polymerization;uses carrier molecules to activate monomersuses carrier molecules to activate monomers
MODULAR DESIGN
SIMPLICITY AND VERSATILITYASSEMBLY-LINE MENTALITY
Don’t have to make every structure from scratch
Simplified chemistry, repeating link Dehydration Synthesis

DehydrationSynthesis
make bytaking water away
H-XXXX-OH H-YYY-OH H-ZZZZZ-OH
H-XXXX- YYY-ZZZZZ-OH
HOH HOH
Hydrolysisdeath by water
Monomers
Polymer
Endless variety of Polymers
Order of MonomersDifferent Amounts of each monomer
H-YYY-XXXX- ZZZZZ-OH
H-XXXX- ZZZZZ- YYY-OH
H-XXXX- YYY-ZZZZZ-OH
H-ZZZZZ- YYY-ZZZZZ-OH
• Monomers form larger molecules by condensation reactions called dehydration reactions
(a) Dehydration reaction in the synthesis of a polymer
HO H1 2 3 HO
HO H1 2 3 4
H
H2O
Short polymer Unlinked monomer
Longer polymer
Dehydration removes a watermolecule, forming a new bond
Figure 5.2A
• Polymers can disassemble by– Hydrolysis
(b) Hydrolysis of a polymer
HO 1 2 3 H
HO H1 2 3 4
H2O
HHO
Hydrolysis adds a watermolecule, breaking a bond
Figure 5.2B

Monomers PolymersCARBOHYDRATESCARBOHYDRATES
Sugars and Sugar DerivativesSugars and Sugar Derivatives
Monosaccharides Polysaccharides
Simple Sugars
GlucoseFructose
Ribose
storagestarch: amylose
amylopectinglycogen
structureFiber: cellulose
Monomers: Monomers: Polymers: Polymers:
Long chains of monomers
OligosaccharidesOligosaccharidesInformational structuresInformational structures
MONOSACCHARIDES = Carbohydrate Monomers
•1 Carbonyl - aldehyde or ketone R-C-H R1-C-R2
==O O
•All Other CARBONSeach have ONE alcohol group
R-OH
Expect them to be HYDROPHILICExpect them to be HYDROPHILIC
AldoAldosugarsugar
KetoKetosugarsugar
Triose sugars(C3H6O3)
Pentose sugars(C5H10O5)
Hexose sugars(C6H12O6)
H C OH
H C OH
H C OH
H C OH
H C OH
H C OH
HO C H
H C OH
H C OH
H C OH
H C OH
HO C H
HO C H
H C OH
H C OH
H C OH
H C OH
H C OH
H C OH
H C OH
H C OH
H C OH
C OC O
H C OH
H C OH
H C OH
HO C H
H C OH
C O
H
H
H
H H H
H
H H H H
H
H H
C C C COOOO
Ald
oses
Glyceraldehyde
RiboseGlucose Galactose
Dihydroxyacetone
Ribulose
Ket
oses
FructoseFigure 5.3
Monosaccharides Vary in length3, 4, 5, 6 or 7 carbons

Carbon with Carbon with 4 different functional groups 4 different functional groups ChiralChiral or asymmetric carbonor asymmetric carbon = = “handed” carbon“handed” carbon
Also differ by Also differ by SPATIAL GEOMETRYSPATIAL GEOMETRY
RightRighthandedhanded
“D” form“D” form
LeftLefthandedhanded“L” form“L” form
StereoisomersStereoisomers not the same not the same
Plane Plane of of
symmetrysymmetry
Not Not chiralchiral
ChiralChiral
Not Not chiralchiral
ChiralChiral
ChiralChiral
ChiralChiral
Triose sugars(C3H6O3)
Pentose sugars(C5H10O5)
Hexose sugars(C6H12O6)
H C OH
H C OH
H C OH
H C OH
H C OH
H C OH
HO C H
H C OH
H C OH
H C OH
H C OH
HO C H
HO C H
H C OH
H C OH
H C OH
H C OH
H C OH
H C OH
H C OH
H C OH
H C OH
C OC O
H C OH
H C OH
H C OH
HO C H
H C OH
C O
H
H
H
H H H
H
H H H H
H
H H
C C C COOOO
Ald
oses
Glyceraldehyde
RiboseGlucose Galactose
Dihydroxyacetone
Ribulose
Ket
oses
FructoseFigure 5.3
Spatial Geometry yields a variety of forms
8 8 Forms!Forms!

5 and 6 Carbon Sugars CIRCULARIZE in WaterTo FORM RINGS
FischerFischerprojectionprojection
HaworthHaworthprojectionprojection
H
H C OH
HO C H
H C OH
H C OH
H C
O
C
H
1
2
3
4
5
6
H
OH
4C
6CH2OH 6CH2OH
5C
HOH
C
H OH
H
2 C
1C
H
O
H
OH
4C
5C
3 C
H
HOH
OH
H
2C
1 C
OH
H
CH2OH
H
H
OHHO
H
OH
OH
H5
3 2
4
(a) Linear and ring forms. Chemical equilibrium between the linear and ringstructures greatly favors the formation of rings. To form the glucose ring,carbon 1 bonds to the oxygen attached to carbon 5.
OH3
O H OO
6
1
Figure 5.4
Circularization causes another Circularization causes another chiralchiral carboncarbon
αα--DD--GlucoseGlucose ββ--DD--GlucoseGlucose
Dehydration reaction in the synthesis of maltose. The bonding of two glucose units forms maltose. The glycosidic link joins the number 1 carbon of one glucose to the number 4 carbon of the second glucose. Joining the glucose monomers in a different way would result in a different disaccharide.
Dehydration reaction in the synthesis of sucrose. Sucrose is a disaccharide formed from glucose and fructose.Notice that fructose,though a hexose like glucose, forms a five-sided ring.
(a)
(b)
H
HO
H
HOH H
OH
O H
OH
CH2OH
H
HO
H
HOH H
OH
O H
OH
CH2OH
H
O
H
HOH H
OH
O H
OH
CH2OH
H
H2O
H2O
H
H
O
H
HOH
OH
O HCH2OH
CH2OH HO
OHH
CH2OH
HOH H
H
HO
OHH
CH2OH
HOH H
O
O H
OHH
CH2OH
HOH H
O
HOH
CH2OH
H HO
O
CH2OH
H
H
OH
O
O
1 2
1 41–4
glycosidiclinkage
1–2glycosidic
linkage
Glucose
Glucose Glucose
Fructose
Maltose
Sucrose
OH
H
H
Figure 5.5
monomeric sugars coupled together by
CONDENSATION REACTION
GlycosidicGlycosidicbondbond
Holds carbohydrates togetherHolds carbohydrates together

BreakdownBreakdownDoes notDoes notRequireRequireEnergyEnergyInputInput
SynthesisSynthesisRequires Requires EnergyEnergyInputInput
Sucrose (glucose+ fructose) Cane SugarLactose (glucose+galactose) Milk SugarMaltose (glucose+glucose) Beer
Dextran (short chain of glucose) Digested Starch
Furans (short chain of fructose) Onions
OligoSaccharides
DiSaccharides
Disaccharides, Oligosaccharides and Polysaccharides(two)(two) (few)(few) (many)(many)
Polysaccharides
•Long chains of Millions of monomers
•most common polymers made ONLY of GLUCOSE monomers
•Storage reserves: •Starch amylose• amylopectin, glycogen
•Structure: cellulose
Chloroplast Starch
Amylose Amylopectin
1 µm
(a) Starch: a plant polysaccharideFigure 5.6
MitochondriaGiycogen granules
0.5 µm
(b) Glycogen: an animal polysaccharide
Glycogen
Figure 5.6

Glycogen (or Glycogen (or AmylopectinAmylopectin))
Polysaccharides of glucose chains inPolysaccharides of glucose chains inan a(1an a(1-->4) linkage, with a(1>4) linkage, with a(1-->6) >6) branchesbranches
Structural Polysaccharides
•• CelluloseCellulose– Is also a polymer of glucose
– But has different glycosidic linkages than starch
– We can readily digest starchesbut cannot digest cellulose
• Cellulose is indigestable to animals– Cows and termites have microbes in
their stomachs to facilitate this process
Figure 5.9 (c) Cellulose: 1– 4 linkage of β glucose monomers
H O
O
CH2OH
HOH H
H
OH
OHH
H
HO
4
C
C
C
C
C
C
H
H
H
HO
OH
H
OH
OH
OH
H
O
CH2OH
HH
H
OH
OHH
H
HO4 OH
CH2OHO
OH
OH
HO41
O
CH2OH
O
OH
OH
O
CH2OH
O
OH
OH
CH2OH
O
OH
OH
O O
CH2OHO
OH
OH
HO4
O1
OH
O
OH OHO
CH2OHO
OH
O OH
O
OH
OH
(a) α and β glucose ring structures
(b) Starch: 1– 4 linkage of α glucose monomers
1
α glucose β glucose
CH2OH CH2OH
1 4 41 1
Figure 5.7 A–C
Starches:αα glycosidic linkage
OH “down”
Cellulose:ββ glycosidic linkage
OH “up”

Cellulose ß(1Cellulose ß(1-->4) linkage>4) linkageAmyloseAmylose a(1a(1-->4) linkage>4) linkage
Plant cells
0.5 µm
Cell walls
Cellulose microfibrilsin a plant cell wall
∩
Microfibril
CH2OH
CH2OH
OHOH
OO
OHOCH2OH
OO
OHO
CH2OH OH
OH OHO
O
CH2OHO
OOH
CH2OH
OO
OH
O
O
CH2OHOH
CH2OHOHOOH OH OH OH
O
OH OH
CH2OH
CH2OH
OHO
OH CH2OH
OO
OH CH2OH
OH
β Glucose monomer
O
O
O
O
O
O
Parallel cellulose molecules areheld together by hydrogenbonds between hydroxyl
groups attached to carbonatoms 3 and 6.
About 80 cellulosemolecules associate
to form a microfibril, themain architectural unitof the plant cell wall.
A cellulose moleculeis an unbranched βglucose polymer.
OH
OH
O
OOH
Cellulosemolecules
Figure 5.8
Polysaccharides Polysaccharides although although hydrophillichydrophillic
arearegenerally generally
Insoluble in waterInsoluble in water
--orders too much water around polymerorders too much water around polymer--Polymer tends to hydrogen bond to itselfPolymer tends to hydrogen bond to itself--Polymer falls out of solutionPolymer falls out of solution
Starch
““polymer effect”polymer effect”
Polymer forms Polymer forms Secondary StructuresSecondary Structures
Polymer hydrogen bonding Polymer hydrogen bonding to to Itself Itself

If If Denature Denature Secondary StructureSecondary Structure
(Break Hydrogen Bonds of Polymer with Itself)(Break Hydrogen Bonds of Polymer with Itself)
Water will Hydrogen bondWater will Hydrogen bondWith PolymerWith Polymer
RESULT IS BOUND WATERRESULT IS BOUND WATER GELGEL
Can FORCE polymer to stay Hydrated Can FORCE polymer to stay Hydrated Sugar Sugar DerrivativesDerrivatives
DisruptDisruptSecondary Secondary StructuresStructures--remain remain Hydrated!Hydrated!
Characteristics?Characteristics?
Some OtherSugar Derivatives or Modified Sugars
Missing one or more components:
a. 5 carbon RIBOSE andDEOXYRIBOSE
missing one alcohol
b. Glycerol - 3 Carbon Sugar withalcohol in place of an aldehyde
c. Sugar amines, Sugar acidshave amine or carboxylic acid group or something else in place of an alcohol
H - C - C - C - H- --
-- -H H H
OH
OH
OH
HHa.a.
b.b.
c.c.
Questions?Questions?

LIPIDSLIPIDShydrophobic characterhydrophobic character
Triglycerides Phospholipids Steroids
“Other”
FATSOILS-long termstorage depot
MEMBRANESMembranes
Hormones
Fatty Acids and Glycerol
Fatty Acid: carboxylic acid with LONG hydrocarbon chainF.A differ by:F.A differ by:
ChainChainlengthlength
saturationsaturation
• Fats– Are constructed from two types of smaller
molecules, a single glycerol and usually three fatty acids
(b) Fat molecule (triacylglycerol)
H HH H
HH H H H H H HH
H HHO
H O HC
C
C
H
H OH
OH
H
HH
HH
H H HH
H H H H HH
H
HCCCCCCCCC
CC
CC
CC C
Glycerol
Fatty acid(palmitic acid)
HH
H
H
H H HH
HH
HH
HH H H H
H H H
HHHHHHHHHHHHHHHH
H
H H H H H H H H H H H H H H HH
HHHHHHHHHHHHHH
H H H H H H H H H H H H H H HH
HHHHHHHHHHHHHHH
HO
OO
O
OC
C
C C C C C C C C C C C C C C C C C
C
CCCCCCCCCCCCCCCC
C C C C C C C C C C C C C C CO
O
(a) Dehydration reaction in the synthesis of a fatEster linkage
Figure 5.11
Triglycerides: 3 fatty acids
linked to Glycerolby CONDENSATION
SYNTHESIS
ESTER LinkageESTER Linkage

Properties in WaterProperties in Water
Insoluble !Insoluble !AllAll
hydrophobichydrophobic
TriglyceridesTriglycerides FATS OILSSolid Liquid
WHY?
SaturatedUnsaturated orPolyunsaturated
Like Fig 3-28
• Saturated fatty acids– Have the maximum number of hydrogen atoms
possible– Have no double bonds
(a) Saturated fat and fatty acid
Stearic acid
Figure 5.12Stack nicelyStack nicely
• Unsaturated fatty acids– Have one or more double bonds
(b) Unsaturated fat and fatty acidcis double bondcauses bending
Oleic acid
Figure 5.12
Do not Stack wellDo not Stack well

Free Fatty Acids
Hydrolyzed Triglycerides
PolarPolar(charged)(charged)HeadHead
Very Very HydrophobicHydrophobicTailTail micellemicelle
FattyFattyAcidsAcids
monolayermonolayer
amphipathicamphipathic
PhospholipidsPhospholipids
NonpolarPolar
Fatty acid tailsFatty acid tailsGl
ycer
ol
PhosphateHeadGroup
Fig 3-27Glycerol linked to 2 fatty acids
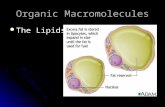
• Phospholipid structure– Consists of a hydrophilic “head” and
hydrophobic “tails”
CH2
OPO OO
CH2CHCH2
OO
C O C O
Phosphate
Glycerol
(a) Structural formula (b) Space-filling model
Fatty acids
(c) Phospholipidsymbol
Hyd
roph
o bic
tails
Hydrophilichead
Hydrophobictails
–
Hyd
roph
i lic
hea d CH2 Choline
+
Figure 5.13
N(CH3)3

PhospholipidPhospholipid Head GroupsHead Groups
HydrophillicHydrophillic! Polar groups! Polar groups
Phospholipid Phospholipid BilayerBilayer
Form Form BoundariesBoundaries
Hydrophilichead
WATER
WATER
Hydrophobictail
Figure 5.14
NonpolarNonpolar FA tailsFA tails
Polar Polar HeadgroupsHeadgroups
Polar Polar HeadgroupsHeadgroups3-D Ball (Sphere)
Vesicle or
Liposome Crosssection
Sheet
Inside
Outside

Hormones:Hormones:Signal moleculesSignal molecules
SummarySummary••Principles of Building PolymersPrinciples of Building Polymers
Directional assembly from simple unitsDirectional assembly from simple unitsRequires energy inputRequires energy inputCondensation dehydration reactionsCondensation dehydration reactions
••CarbohydratesCarbohydratesmonosaccharidesmonosaccharidespolysaccharidespolysaccharides
••LipidsLipidsTriglyceridesTriglyceridesphospholipidsphospholipidssteroidssteroids
Next Time:
More Macromolecules
Proteins Proteins
Nucleic AcidsNucleic Acids