1q21/1p21 in the Evaluation of Patients with Multiple...
Transcript of 1q21/1p21 in the Evaluation of Patients with Multiple...

TM
Volume 17 No.3Summer 2014Laboratories 1-888-92-PHENO www.phenopath.com
The hallmark of Lynch Syndrome is a germ line mutation in one of the enzymes involved in DNA mismatch repair (MMR). The diagnosis of Lynch Syndrome is of particular relevance in colorectal and endometrial cancer cases, and several testing modalities including IHC, PCR, methylation studies, and genetic sequence analy-sis are used for its evaluation. As many sporadic cancers exhibit MMR deficiency, the goal of current testing algorithms is to identify such pa-tients so that genetic sequence testing is performed only when Lynch Syndrome remains a reasonable possibility.
PhenoPath’s testing algorithm for Lynch Syndrome is based on the most recent National Comprehensive Cancer Network (NCCN) consensus guidelines and is shown in the diagram to the right. Important high-lights of this algorithm include:
1. MMR/Microsatellite instability testing should be performed on ALL colorectal cancer patients < 70 years of age (previously < 50 years of age) and also those ≥70 years old who meet Bethesda Guidelines.
2. In colorectal cancer, BRAF V600 mutation testing is recommended if the tumor shows loss of MLH1. The presence of a BRAF mutation indicates that the loss of MLH1 expression is likely due to methyla-tion of the MLH1 promoter not due to a germline mutation, argu-ing against Lynch Syndrome.
3. In endometrial cancers, BRAF V600 mutation testing is not currently indicated. BRAF testing is also not indicated when loss of MSH2 and/or MSH6 is observed.
4. Results of any testing should be correlated clinically, and genetic counseling should be considered where appropriate.
The testing algorithm is available from Client Services. Given the clini-cal implications of Lynch testing, our pathologists are available for dis-cussion and to answer questions.
PhenoPath UpdatesRecommended Lynch Syndrome Algorithm
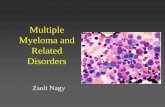
PhenoPath is pleased to announce the 1q21/1p21 prognostic FISH assay for multiple myeloma. In multiple myeloma, genetic alterations involving chromosome 1 have been found to be associated with advanced stage disease and poor prognosis. The 1q21/1p21 FISH assay detects 1q21 amplifications (see image) and 1p21 deletions. In recent studies, these chromosome 1 alterations have been described in up to 43% of multiple myeloma patients. More specifically, studies show that 1q gains or 1p deletion are risk factors for poor outcome after intensive therapy for multiple myeloma. Therefore, use of this FISH assay can aid in the evaluation of patients with multiple myeloma. Please contact Client Services for details ([email protected]). References:1. Hanamura I et al. Blood 108:1724-1732, 2006.2. Marzin Y et al. Anticancer Research 26:953-959, 2006.3. Chang H et al. Bone Marrow Transplant 45:117-121, 2010.4. Sonneveld, Pieter. Blood 108:1426-1427, 2006.
1q21/1p21 in the Evaluation of Patients with Multiple Myeloma
1q gain & 1p normal
Lynch Syndrome Screening
Loss of MSH2/MSH6(or all 4 markers)
MMR by IHC (MLH1, MSH2, MSH6, PMS2)
Clinical assessment and/or Bethesda criteria
supports MSI testing
Consider:Germline MMR
gene sequencing and EPCAM genetic testing with
genetic counseling
Consider:Germline MMR
gene sequencing withgenetic counseling
STOP(if strong clinical
suspicion for Lynch Syndrome, consider
MSI PCR testing)
STOP = No further testing recommended, unless significant clinical suspicion for Lynch Syndrome
BRAF V600 by PCR
MLH1 Methylation
Loss of expression (MLH1/PMS2)
Positive
Colon carcinoma
Positive
No loss of expression
Negative
Negative
STOP
Endometrial carcinoma
STOP

IHC Breast Multiplex (Keratins 5/14 + P63 + Keratins 7/18) The IHC Breast Multiplex assay is intended for the qualitative identification of keratin 5/14, p63 protein and keratin 7/18 by two-color IHC. This multiplex assay has two diagnostic applications:
1. Invasive carcinoma versus non-invasive lesions: The presence of myoepithelial cells around suspicious cell nests argues against a diagnosis of invasive carcinoma and can aid in identifying a non-invasive entity, such as carci-noma in situ, atypical ductal hyperplasia (ADH), usual ductal hyperplasia (UDH), and sclerosing adenosis.
2. Usual ductal hyperplasia versus atypical ductal hyperplasia and carcinoma in situ: The presence of staining for keratin 5 among the luminal cells of a hyperplastic breast lesion can aid in distinguishing usual ductal hyperpla-sia (typically positive for keratin 5) from atypical ductal hyperplasia/carcinoma in situ (typically negative for keratin 5).
IHC Prostate Multiplex (Keratins 5/14 + P63 + P504S)The IHC Prostate Multiplex assay is intended for the qualitative identification of keratin 5/14, p63 protein and P504S by two-color IHC.
Keratin 5 and keratin 14 are expressed in the basal cells of normal prostate glands and prostatic intraepithelial neoplasia (PIN), a precursor lesion to prostatic adenocarcinoma; however, expression of keratin 5 or keratin 14 is not seen in invasive prostatic adenocarcinoma. p63 is detected in nuclei of the basal epithelium in normal prostate glands and PIN, and is not seen in the vast majority of adenocarcinomas of the prostate.
Alpha-Methyl Acyl-CoA-Racemase (AMACR or racemase) is the gene product of P504S, a peroxisomal and mitochondrial enzyme that plays a role in bile acid synthesis and oxidation of branched chain fatty acids. By immunohistochemistry, AMACR/P504S is very frequently expressed in prostatic adenocarcinoma. Prostate glands involved in PIN have also been found to express AMACR/P504S, whereas in most cases, AMACR/P504S is negative in benign glands.
Together, the presence of racemase expression in atypical glands, and the absence of basal cells (negative staining for p63, keratins 5 and 14) can aid in the diagnosis of prostatic adenocarcinoma.
A recent collaborative study between PhenoPath (Dr. Harry Hwang, Dr. Christopher Tse, Stephanie Rodriguez, and Dr. Allen Gown) and Dr. Andy Churg (University of British Columbia Department of Pathology) on p16 FISH in mesothelial surface proliferations was published in the May issue of American Journal of Surgical Pathology (Hwang et al., AJSP 38:681-688, 2014). Homozygous deletion of p16INK4a has been described as a marker of malignant mesothelioma, and while relatively insensitive, such loss has been demonstrated to be very specific for the diagnosis of mesothelioma. While published literature has addressed homozygous p16 loss in invasive lesions, the goal of this study was to determine if evaluation for p16 homozygous loss by FISH on small biopsies containing atypical surface mesothelial proliferations could be predictive of an underlying mesothelioma. Specifically, this study shows that there was perfect correlation for p16 gene deletion status between atypical surface proliferations and the underlying invasive mesothelioma. This finding has particular implications in the management of patients with radiologic or surgical evidence of a pleural or peritoneal tumor, especially when the tissue biopsy shows only a surface mesothelial proliferation. In these cases, based on our study, p16 FISH can be used to either help establish the diagnosis of mesothelioma or need for rebiopsy. This study therefore expands the utility of p16 gene status by FISH in the clinicopathologic management of patients with pleural or peritoneal tumors.
AJSP Publication on New Marker of Malignant Mesothelioma
PhenoPath Introduces Multiplex AssaysPhenoPath is pleased to announce the release of two immunohistochemistry (IHC) multiplex assays, whereby multiple markers are identified on a single slide. These assays are particularly useful in difficult cases with limited tissues.
Prostate Multiplex
Breast Multiplex
p16 FISH Deletion in Surface Epithelial Mesothelial
Proliferations Is Predictive of Underlying InvasiveMesothelioma
Harry Hwang, MD,* Christopher Tse, MBBS,* Stephanie Rodriguez, HT, ASCP,*
Allen Gown, MD,* and Andrew Churg, MDwAbstract: An atypical mesothelial proliferation along the pleural
or peritoneal surface without evidence of invasive tumor poses a
diagnostic challenge. Homozygous deletion of p16 (CDKN2A)
by fluorescence in situ hybridization (FISH) has been shown to
be a good marker of malignancy in mesothelial proliferations,
but correlations of p16 status between atypical surface pro-
liferations and underlying mesothelioma have not been de-
scribed. We used p16 FISH to investigate 11 pleural and 7
peritoneal mesotheliomas that had both an invasive component
and a separate surface mesothelial proliferation. In 5/11 pleural
samples and 1/7 peritoneal samples, the invasive mesotheliomas
showed homozygous deletion of p16 (all cases in excess of 90%
of cells deleted); the surface proliferation in all 6 cases with
deletion in the invasive tumor was also p16 deleted. Conversely,
the 12 tumors that did not show p16 deletion in the invasive
compartment also did not have deletion in the surface compo-
nent. We conclude that (1) surface mesothelial proliferations
near invasive mesotheliomas show the same pattern of p16 by
FISH as the underlying tumor and may represent in situ disease
or growth of the underlying mesothelioma along the serosal
surface; (2) p16 deletion in mesothelial surface proliferations is
strongly associated with p16 deletion in underlying meso-
theliomas, and biopsies consisting of pure surface mesothelial
proliferations that are p16 deleted allow a diagnosis of meso-
thelioma without an additional biopsy if there is clinical (thor-
acosopic/laparoscopic) or radiologic evidence of diffuse pleural
or peritoneal tumor; (3) however, the absence of p16 deletion in
surface proliferations does not rule out underlying invasive
mesothelioma.Key Words: malignant mesothelioma, atypical mesothelial pro-
liferation, p16 FISH(Am J Surg Pathol 2014;38:681–688)
S mall pleural and peritoneal biopsies not infrequently
show proliferations of mesothelial cells that are wor-
risome for, but not diagnostic, of malignancy, sometimes
because of cytologic features but more often because of
the pattern of growth.1,2 One the most difficult patterns in
such specimens is mesothelial proliferations that are
confined to the pleural or peritoneal surface. These pro-
liferations can appear as a single line of mesothelial cells
but may also grow in more complex patterns and/or be
cytologically very atypical; however, the current recom-
mendation is not to diagnose malignant mesothelioma
in pure surface proliferations, because even unusual-
appearing mesothelial surface proliferations may turn out
to be benign reactions. Indeed, there are no morphologic
features that reliably separate benign from malignant in
this setting.1–3 At present, a biopsy showing only a sur-
face mesothelial proliferation is nondiagnostic, even if
there is radiologic or clinical (thoracoscopic/laparoscopic)
evidence of tumor. In this situation an additional biopsy
is required for a tissue diagnosis.Because of the limitations of standard histologic
evaluation, a variety of immunohistochemical markers
have been proposed for separating benign from malignant
mesothelial processes, but in our opinion they are un-
reliable (see the Discussion section). More recently, in-
vestigators have studied the potential of p16INK4a(also
called CNKN2A) as another marker in this diagnostic
area. p16INK4ais a cyclin-dependent kinase inhibitor and
a member of the INK4 (INhibitor of CDK4) family of
cell cycle regulatory proteins that bind and generally in-
hibit D-type cyclin-dependent kinases. Homozygous de-
letion of the p16 gene locus has been described in a
number of human tumors including carcinomas primary
to the bladder, breast, and prostate. Recent studies have
shown that some proportion of mesotheliomas demon-
strate homozygous deletion of p16 when assayed by flu-
orescence in situ hybridization (FISH), either using
cytology preparations or tissue biopsies.4–9
In this study we have used p16 FISH testing on a
series of pleural and peritoneal mesotheliomas that, on
biopsy, have a surface mesothelial proliferation as well as
an invasive component to determine whether surface
proliferations ever show p16 deletion and, if so, whether
that finding correlates with p16 deletion in the underlying
mesothelioma.
From the *PhenoPath Laboratories, Seattle, WA; and wDepartment of
Pathology, Vancouver General Hospital and University of British
Columbia, Vancouver, BC, Canada.
Conflicts of Interest and Source of Funding: The authors have disclosed
that they have no significant relationships with, or financial interest
in, any commercial companies pertaining to this article.
Correspondence: Andrew Churg, MD, Department of Pathology, Van-
couver General Hospital, 910 W 10th Ave, Vancouver, BC, Canada
V5Z 1M9 (e-mail: achurg@ mail.ubc.ca).
Copyright r 2014 by Lippincott Williams & Wilkins
ORIGINAL ARTICLE
Am J Surg Pathol � Volume 38, Number 5, May 2014
www.ajsp.com | 681

Other recent PhenoPath publications: Lee AF, et al. IMP3 and GLUT-1 Immunohistochemistry for Distinguishing Benign From Malignant Mesothelial Proliferations. AJSP 37:421-6, 2013.In this study (performed in collaboration with Drs. Andrew Churg and Anna Lee at the University of British Columbia), the combined use of IMP3 (insulin-like growth factor II mRNA binding protein 3) and GLUT-1 (glucose transporter protein 1) was able to distinguish benign from malignant mesothelial proliferations.
Oliva E, et al. Immunohistochemistry as an adjunct in the differential diagnosis of radiation-induced atypia versus urothelial carcinoma in situ of the bladder: a study of 45 cases. Hum Pathol 44: 860-6, 2013.This study was a collaboration among pathologists at PhenoPath, Cedars Sinai Medical Center, and Massachusetts General Hospital. In this comprehensive study employing antibodies to keratin 20, p53, and CD44s, the immunophenotype of radiation induced atypia was not found to differ significantly to that described for other kinds of reactive urothelium, and can be used to aid in the distinction of radiation induced atypia from carcinoma in situ.
Husain AN, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma: 2012 Update of the Consensus Statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 137:647-67, 2013.A useful update from the International Mesothelioma Interest Group, of which Dr. Gown is a member, providing an update (as of 2012) of recom-mendations and criteria to assist in the identification of mesothelioma, including important updates on immunohistochemistry.
Blanco LZ Jr, et al. Immunohistochemical characterization of squamous differentiation and morular metaplasia in uterine endometrioid adenocarcinoma. Int J Gynecol Pathol 32(3):283-92, 2013.This study was a collaboration between pathologists at PhenoPath and Rush University Medical Center. Using a panel of 8 different markers, including PAX8, p16, p63 and ER, the immunophenotype of squamous differentiation and modular metaplasia, both of which are frequently present in uterine endometrioid adenocarcinoma, was contrasted and further distinguished from adenocarcinoma and normal endometrium.
SF-1SF-1 (steroidogenic factor 1) is a nuclear transcription factor that controls key genes involved in sexual develop-ment and reproduction. These include many genes involved in gonadal and adrenal steroidogenesis. As such, in normal tissues, SF-1 is expressed in Sertoli cells of the testis and adrenal cortical cells. In studies published over the past few years, SF-1 has proven to be a highly sensitive and specific marker for the identification of sex cord stromal tumors in both the ovary and testis. For example, SF-1 is expressed in Sertoli tumors of the ovary, but not in endometrioid tumors or carcinoids, two tumors which are often in the differential diagnosis of Sertoli tumors. SF-1 has proved more sensitive for the identification of ovarian sex cord stromal tumors (including granulosa cell, Sertoli cell, Sertoli-Leydig, steroid cell, and fibroma/fibrothecoma) than previous markers such as WT-1, CD99, calretinin, MART-1, and inhibin alpha. In contrast, SF-1 is not expressed in some tumors which can show histologic overlap with sex cord stromal tumors, such as germ cell tumors, including seminoma, embryonal carcinoma, and yolk sac tumor. And as a bonus, SF-1 can also be employed to help identify adrenal cortical tumors and distinguish them from pheochromocytomas or renal cell carcinomas.
CD19CD19 is a pan-B-lineage antigen that, like CD79a, is first expressed in very early B-lymphoid progenitors, and continues to be expressed at variable levels through terminal B-lymphoid differentiation in plasma cells. CD19 shows mainly membranous expression, and a smaller amount of cytoplasmic expression. With the exception of true plasma cell neoplasms and classical Hodgkin lymphoma, the great majority of mature and immature B-lym-phoid neoplasms express CD19. Some neoplasms such as hairy cell leukemia overexpress CD19, while others such as follicular lymphoma typically underexpress CD19. Rare non-B-lymphoid neoplasms express CD19, most notably acute myeloid leukemia (AML) bearing the t(8;21).
Granzyme B Granzyme B is a serine protease that is the main component in granule-mediated targeted cell lysis. Cytotoxic T-lymphocytes (CTL) and natural killer (NK) cells store granzyme B-containing granules in their cytoplasm and release them during CTL-mediated targeted cell lysis (the ‘cell-mediated’ component of the immune response). After release from the CTL, granzyme B binds its receptor on the target cell, is endocytosed, and remains in the endocytic vesicle until released by perforin. Once released into the target cell cytoplasm, granzyme B can effect cell death via two pathways: 1) initiating a caspase cascade that leads to rapid DNA fragmentation of the target cell (apoptosis) or 2) mediating the release of cytochrome c from the mitochondria, which leads to cell death via necrosis. Granzyme B is, therefore, a useful immunohistochemical marker for activated cytotoxic T-lymphocytes
and NK cells. In addition, granzyme B may aid in differentiating neoplasms arising from these cytotoxic cell subtypes and neoplasms arising from other hematolymphoid lineages. Note that granzyme B can be detected at high levels only in activated cytotoxic cells, unlike T-cell intracellular antigen-1 (TIA-1), which can be detected in all cytotoxic cells regardless of activation state.
References: 1. Sangoi AR et al. Appl Immunohistochem Mol Morphol 21:318-21, 2013. 2. Zhao C et al. Int J Gynecol Pathol 27:507-14, 2008. 3. Zhao C et al. AJSP 33:354-66, 2009. 4. Sangoi AR et al. AJSP 34:423-32, 2010. 5. Enriquez ML et al. Appl Immunohistochem Mol Morphol 20:141-5, 2012.
Latest and Greatest IHC Markers

551 North 34th Street, Suite100Seattle, Washington, 98103P (206) 374-9000 F (206) 374-9009
PhenoPathDiagnoses you can count on®
FEATURED At the Next PhenoPath ConferenceRichard Zaino, MD Penn State M.S. Hershey Medical CenterPhenoPath, Thursday, September 18, 2014, 6:30 PM (light dinner), 7:30 PM (talk)
Richard Zaino, MD will present “Diagnostic Dilemmas Involving the Endocervix” at the PhenoPath Summer Conference at 7:30 PM on Thursday, September 18, 2014. Dr. Zaino will also give a daytime lecture, “Hydatidiform Mole and its Mimics” at noon the same day.
Richard Zaino, MD is a Professor of Pathology at Penn State M.S. Hershey Medical Center, where he has been a faculty member since 1980. He attended medical school and completed his AP/CP residency at Duke University, following which he entered into a rural community practice in the Smokey Mountains. He shortly thereafter returned to academics, where he has practiced cytology, autopsy and surgical pathology, with a subspecialty interest in gynecologic pathology. His research focus is directed at the cyclic growth and regulation
of differentiation of the normal endometrium and diseases that result from its dysregulation. He has published about 130 scientific articles, 20 book chapters, and one book. He directed the Pathology Residency Program for 17 years and was Chief of the Division of Anatomic Pathology. He has had a long interest in medical education, and has been recognized with more than 20 teaching awards at Hershey Medical Center, where he was selected as an inaugural member of the Distinguished Educators Society. He developed a short course on effective teaching strategies that has been presented at the USCAP. He has been an active member of the Gynecologic Oncology Group, where he chaired the Pathology Committee for 10 years, and co-chaired the Committee on the Uterine Corpus. He has served on multiple journal editorial boards, international societies and national advisory committees.