1.cdn.edl.io Web viewVocabulary Word: Definition; Scientific Notation. Accuracy. Precision. ......
Transcript of 1.cdn.edl.io Web viewVocabulary Word: Definition; Scientific Notation. Accuracy. Precision. ......

Using and Expressing Scientific NotationIn science, measurements must be accurate, precise, and written to the correct number of significant figures. Use chapter 3 (pages 62-71) of your textbook to answer the following questions.
Vocabulary1. Define the following terms:
Vocabulary Word Definition
Scientific Notation
Accuracy
Precision
Percent error
Significant figure
Scientific Notation2. Why are numbers used in chemistry expressed in scientific notation?
3. What form does scientific notation always take the form of?
4. What are the steps to putting a number into scientific notation?
5. Convert the following to scientific notation:
a. 8,790,000,000
b. .000458
6. Use your calculator to perform the following operations with scientific notation.
a. (4.3 x 104) + (3.9 x 103) =
b. (8.0 x 104) x (5.0 x 102) =
c. (6.9 x 107) / (3.0 x 10-5) =

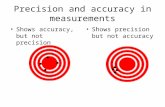
Accuracy, Precision, and Error - Accuracy, precision, and error help determine the reliability of measurements.
7. Place dots on the targets below to illustrate the following:Low accuracyLow precision
Low accuracyHigh precision
High accuracyLow precision
High accuracyHigh precision
Significant Figures Rules
1. All nonzero numbers are significant.
2. Zeros between two numbers are significant
3. Zeros to the left of the first nonzero number are not significant
4. Zeros to the right of a nonzero number after the decimal are significant.
5. Zeros to the right a of a nonzero number before the decimal are significant. Zeros before an understood decimal are placeholders and are not significant.
8. Determine how many significant figures are in each of the following numbers.
a. 5.703
b. 70
c. 100.
d. 395830
e. 0.0101
f. 21.0