16. Mixtures
Transcript of 16. Mixtures

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 1/77
© 2012 Pearson Education, Inc.
Conceptual
PhysicalScience5th Edition
Chapter 16:
MIXTURES
© 2012 Pearson Education, Inc.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 2/77
© 2012 Pearson Education, Inc.
This lecture will help you
understand:
• Most Materials are Mixtures
• The Chemist’s Classification of Matter
• Solutions
• Solubility• Soaps and Detergents
• Softening Hard Water
• Purifying the Water We Drink
• Wastewater Treatment

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 3/77
© 2012 Pearson Education, Inc.
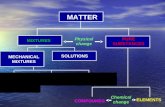
Most Materials are Mixtures
• Pure substance
A material consisting of only one type of element or compound.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 4/77
© 2012 Pearson Education, Inc.
• Pure substance
A material consisting of only one type of element or compound.
• Mixture
A collection of two or more pure substances.
Most Materials are Mixtures

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 5/77
© 2012 Pearson Education, Inc.
• Pure substance
A material consisting of only one type of element or compound.
• Mixture
A collection of two or more pure substances.
—can be separated by physical means
Most Materials are Mixtures

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 6/77
© 2012 Pearson Education, Inc.
Most Materials are Mixtures

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 7/77© 2012 Pearson Education, Inc.
The Chemist’s Classification of
Matter

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 8/77© 2012 Pearson Education, Inc.
The Chemist’s Classification of
Matter
• Pure materials consist of a single element
or compound.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 9/77© 2012 Pearson Education, Inc.
The Chemist’s Classification of
Matter
• Pure materials consist of a single element
or compound.
• Impure materials consist of two or more
elements or compounds.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 10/77© 2012 Pearson Education, Inc.
The Chemist’s Classification of
Matter
• Pure materials consist of a single element
or compound.
• Impure materials consist of two or more
elements or compounds.
• Mixtures may be heterogeneous or
homogeneous.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 11/77© 2012 Pearson Education, Inc.
• In heterogeneous mixtures, the different
components can be seen as individual
substances.
The Chemist’s Classification of
Matter

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 12/77© 2012 Pearson Education, Inc.
• In heterogeneous mixtures, the different
components can be seen as individual
substances.
• In homogenous mixtures, the composition
is the same throughout.
The Chemist’s Classification of
Matter

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 13/77© 2012 Pearson Education, Inc.
The Chemist’s Classification of
Matter

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 14/77
© 2012 Pearson Education, Inc.
• Homogeneous mixtures
The Chemist’s Classification of
Matter

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 15/77
© 2012 Pearson Education, Inc.
• Homogeneous mixtures
– Solution: all components in the same phase.
The Chemist’s Classification of
Matter

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 16/77
© 2012 Pearson Education, Inc.
• Homogeneous mixtures
– Solution: all components in the same phase.
– Suspension: different components in different
phases.
The Chemist’s Classification of
Matter
Th Ch i t’ Cl ifi ti f M tt

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 17/77
© 2012 Pearson Education, Inc.
Is the air in your house a homogeneous or a
heterogeneous mixture?
A. Homogeneous, because it is mixed very well.
B. Heterogeneous, because of the dust particles it contains.
C. Homogeneous, because it is all at the same temperature.
D. Heterogeneous, because it consists of different types of
molecules.
The Chemist’s Classification of Matter
CHECK YOUR NEIGHBOR
Th Ch i t’ Cl ifi ti f M tt

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 18/77
© 2012 Pearson Education, Inc.
Is the air in your house a homogeneous or a
heterogeneous mixture?
A. Homogeneous, because it is mixed very well.
B. Heterogeneous, because of the dust particles it contains.
C. Homogeneous, because it is all at the same temperature.
D. Heterogeneous, because it consists of different types of
molecules.
The Chemist’s Classification of Matter
CHECK YOUR ANSWER

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 19/77
© 2012 Pearson Education, Inc.
Solutions
• Solution: A homogenous mixtureconsisting of ions or molecules
• Solvent: The major component of asolution.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 20/77
© 2012 Pearson Education, Inc.
Solutions
• Solution: A homogenous mixtureconsisting of ions or molecules
• Solvent: The major component of asolution.
• Solute: The minor components of a
solution.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 21/77
© 2012 Pearson Education, Inc.
Solutions
• Solution: A homogenous mixtureconsisting of ions or molecules
• Solvent: The major component of asolution.
• Solute: The minor components of a
solution.• Saturated: Said of a solution in which no
more solute will dissolve.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 22/77
© 2012 Pearson Education, Inc.
Solutions
• Concentration: A measure of the amountof solute dissolved in solution.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 23/77
© 2012 Pearson Education, Inc.
Solutions
• Concentration: A measure of the amountof solute dissolved in solution.
Concentration =
Solute
Solution

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 24/77
© 2012 Pearson Education, Inc.
Solutions
• Concentration: A measure of the amountof solute dissolved in solution.
Concentration =Solute
Solution
―concentrated‖

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 25/77
© 2012 Pearson Education, Inc.
Solutions
• Concentration: A measure of the amountof solute dissolved in solution.
Concentration =Solute
Solution

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 26/77
© 2012 Pearson Education, Inc.
Solutions
• Concentration: A measure of the amountof solute dissolved in solution.
Concentration =Solute
―dilute‖
Solution

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 27/77
© 2012 Pearson Education, Inc.
Solutions
• Concentration: A measure of the amountof solute dissolved in solution.
• Mole: A super-large number, 6.02 1023,
used to measure numbers of atoms or molecules, a.k.a. Avogadro’s number.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 28/77
© 2012 Pearson Education, Inc.
Solutions
• Concentration: A measure of the amountof solute dissolved in solution.
• Mole: A super-large number, 6.02 1023,
used to measure numbers of atoms or molecules, a.k.a. Avogadro’s number.
The formula mass of a
substance expressed in
grams contains one mole.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 29/77
© 2012 Pearson Education, Inc.
Solutions
• Concentration: A measure of the amountof solute dissolved in solution.
• Mole: A super-large number, 6.02 1023,
used to measure numbers of atoms or molecules, a.k.a. Avogadro’s number.
The formula mass of a
substance expressed in
grams contains one mole.
Substance Formula Mass
Carbon, C 12
Oxygen, O2 32
Carbon dioxide, CO2 44
Sucrose, C12H22O11 342

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 30/77
© 2012 Pearson Education, Inc.
Solutions
Sucrose, C12H22O11 = 342 g/mole
Solutions

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 31/77
© 2012 Pearson Education, Inc.
Water, H2O, has a formula mass of 18. How many moles of water are there in 18 grams of water?
A. 0.5 moles
B. 1 mole
C. 9 moles
D. 18 moles
Solutions
CHECK YOUR NEIGHBOR
Solutions

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 32/77
© 2012 Pearson Education, Inc.
Water, H2O, has a formula mass of 18. How many moles of water are there in 18 grams of water?
A. 0.5 moles
B. 1 mole
C. 9 moles
D. 18 moles
Solutions
CHECK YOUR ANSWER
Solutions

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 33/77
© 2012 Pearson Education, Inc.
How many grams of water, H2O, are there in 2 moles of water?
A. 1 gram
B. 9 grams
C. 18 grams
D. 36 grams
Solutions
CHECK YOUR NEIGHBOR
Solutions

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 34/77
© 2012 Pearson Education, Inc.
How many grams of water, H2O, are there in 2 moles of water?
A. 1 gram
B. 9 grams
C. 18 grams
D. 36 grams
Solutions
CHECK YOUR ANSWER

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 35/77
© 2012 Pearson Education, Inc.
Solutions
• Molarity: A unit of concentration expressedin moles solute per liter of solution.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 36/77
© 2012 Pearson Education, Inc.
Solutions
• Molarity: A unit of concentration expressedin moles solute per liter of solution.
Molarity =
Moles of Solute
Liters of Solution

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 37/77
© 2012 Pearson Education, Inc.
Solutions
• Molarity: A unit of concentration expressedin moles solute per liter of solution.
• ppm: A unit of concentration expressed in
milligrams solute in liters of solution.
• Molarity: A unit of concentration expressedin moles solute per liter of solution.
• ppm: A unit of concentration expressed in
milligrams solute in liters of solution.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 38/77
© 2012 Pearson Education, Inc.
Solutions
• Molarity: A unit of concentration expressedin moles solute per liter of solution.
• ppm: A unit of concentration expressed in
milligrams solute in liters of solution.
1 ppm =
• Molarity: A unit of concentration expressedin moles solute per liter of solution.
• ppm: A unit of concentration expressed in
milligrams solute in liters of solution.
1 part solute
1,000,000 parts solution
1 milligram solute
1 liter solution=

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 39/77
© 2012 Pearson Education, Inc.
Solubility
• Solubility: The ability of a solute to dissolve
in a solvent.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 40/77
© 2012 Pearson Education, Inc.
Solubility
• Solubility: The ability of a solute to dissolve
in a solvent.
• Soluble: Said of a solute that has
appreciable solubility.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 41/77
© 2012 Pearson Education, Inc.
Solubility
• Precipitate: Solute that comes out of
solution.
Solubility

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 42/77
© 2012 Pearson Education, Inc.
The amount of oxygen, O2, dissolved in the waters of the
arctic ocean is greater, about equal to, or less than the
amount of oxygen dissolved in warm tropical waters? A. Greater than
B. About equal
C. Less than
D. It depends
y
CHECK YOUR NEIGHBOR
Solubility

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 43/77
© 2012 Pearson Education, Inc.
The amount of oxygen, O2, dissolved in the waters of the
Arctic Ocean is greater, about equal to, or less than the
amount of oxygen dissolved in warm tropical waters? A. Greater than
B. About equal
C. Less than
D. It depends
Explanation:
The solubility of oxygen in water decreases with increasing
temperature. As a consequence, cold polar oceans tend to be
more fertile than warmer tropical waters.
y
CHECK YOUR ANSWER
Chapter 12 Review

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 44/77
© 2012 Pearson Education, Inc.
By mass, water is 88.88 percent
oxygen. So why can’t we
breathe water?
p
CHECK YOUR NEIGHBOR
Chapter 12 Review

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 45/77
© 2012 Pearson Education, Inc.
By mass, water is 88.88 percent
oxygen. So why can’t we
breathe water? Hint: What is the elemental formula for the oxygen we breathe and
the chemical formula for water?
p
CHECK YOUR NEIGHBOR

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 46/77
© 2012 Pearson Education, Inc.
Soaps and Detergents
• Soaps and detergents have both polar and
nonpolar properties.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 47/77
© 2012 Pearson Education, Inc.
Soaps and Detergents
• Soaps and detergents have both polar and
nonpolar properties.
• Nonpolar part attracts to the ―grime‖.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 48/77
© 2012 Pearson Education, Inc.
Soaps and Detergents
• Soaps and detergents have both polar and
nonpolar parts.
• Nonpolar part attracts to the ―grime‖.
• Polar part attracts to water.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 49/77
© 2012 Pearson Education, Inc.
Soaps and Detergents
Soap and Detergents

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 50/77
© 2012 Pearson Education, Inc.
Soap attracts ―grime‖ by which type of molecular
interaction?
A. Dipole –dipole.
B. Induced dipole –induced dipole.
C. Hydrogen bonding.
D. Dipole –induced dipole.
CHECK YOUR NEIGHBOR
Soap and Detergents

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 51/77
© 2012 Pearson Education, Inc.
Soap attracts ―grime‖ by which type of molecular
interaction?
A. Dipole –dipole.
B. Induced dipole –induced dipole.
C. Hydrogen bonding.
D. Dipole –induced dipole.
CHECK YOUR ANSWER

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 52/77
© 2012 Pearson Education, Inc.
Softening Hard Water
• Hard water has high concentrations of
calcium and magnesium.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 53/77
© 2012 Pearson Education, Inc.
Softening Hard Water
• Hard water has high concentrations of
calcium and magnesium.
• Undesirable effects

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 54/77
© 2012 Pearson Education, Inc.
Softening Hard Water
• Hard water has high concentrations of
calcium and magnesium.
• Undesirable effects
– Clogged pipes

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 55/77
© 2012 Pearson Education, Inc.
Softening Hard Water
• Hard water has high concentrations of
calcium and magnesium.
• Undesirable effects
– Clogged pipes
– Lower cleaning action of soaps and
detergents

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 56/77
© 2012 Pearson Education, Inc.
Softening Hard Water
• Hard water has high concentrations of
calcium and magnesium.
• Undesirable effects
– Clogged pipes
– Lower cleaning action of soaps and
detergents
– Soap scum

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 57/77
© 2012 Pearson Education, Inc.
Softening Hard Water
• Detergent additives attract the Ca2+ and
Mg2+ ions in hard water.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 58/77
© 2012 Pearson Education, Inc.
Softening Hard Water
• Detergent additives attract the Ca2+ and
Mg2+ ions in hard water.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 59/77
© 2012 Pearson Education, Inc.
Softening Hard Water
• Some homes contain water softening
units.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 60/77
© 2012 Pearson Education, Inc.
Softening Hard Water
• Some homes contain water softening
units.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 61/77
© 2012 Pearson Education, Inc.
Purifying the Water We Drink
• The first step to purifying water is
removing particles and bacteria.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 62/77
© 2012 Pearson Education, Inc.
Purifying the Water We Drink
• The first step to purifying water is
removing particles and bacteria.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 63/77
© 2012 Pearson Education, Inc.
• Water is then aerated to improve the taste
and smell.
Purifying the Water We Drink

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 64/77
© 2012 Pearson Education, Inc.
• Water is then aerated to improve the taste
and smell.
• Lastly, the water is disinfected with
chlorine gas (or ozone).
Purifying the Water We Drink
Purifying the Water We DrinkCHECK YOUR NEIGHBOR

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 65/77
© 2012 Pearson Education, Inc.
Which of the following is not a method of
disinfecting drinking water?
A. Chlorine gas
B. Boiling
C. Aeration
D. Iodine tablets
CHECK YOUR NEIGHBOR
Purifying the Water We DrinkCHECK YOUR NEIGHBOR

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 66/77
© 2012 Pearson Education, Inc.
Which of the following is not a method of
disinfecting drinking water?
A. Chlorine gas
B. Boiling
C. Aeration
D. Iodine tablets
CHECK YOUR NEIGHBOR

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 67/77
© 2012 Pearson Education, Inc.
• Sea water can be converted to drinking
water through desalination.
Purifying the Water We Drink

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 68/77
© 2012 Pearson Education, Inc.
• Sea water can be converted to drinking
water through desalination.
– Distillation
– Reverse osmosis
Purifying the Water We Drink

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 69/77
© 2012 Pearson Education, Inc.
Purifying the Water We Drink

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 70/77
© 2012 Pearson Education, Inc.
Purifying the Water We Drink

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 71/77
© 2012 Pearson Education, Inc.
Purifying the Water We Drink

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 72/77
© 2012 Pearson Education, Inc.
Wastewater Treatment
• Screening removes large insoluble items.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 73/77
© 2012 Pearson Education, Inc.
Wastewater Treatment
• Screening removes large insoluble items.
• Primary treatment allows smaller
insolubles to settle to the bottom or rise to
the top for removal.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 74/77
© 2012 Pearson Education, Inc.
Wastewater Treatment
• Screening removes large insoluble items.
• Primary treatment allows smaller
insolubles to settle to the bottom or rise to
the top for removal.
• Secondary treatment aerates the water
and allows finer particles to settle for
removal.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 75/77
© 2012 Pearson Education, Inc.
Wastewater Treatment
• Screening removes large insoluble items.
• Primary treatment allows smaller
insolubles to settle to the bottom or rise to
the top for removal.
• Secondary treatment aerates the water
and allows finer particles to settle for
removal.
• Tertiary treatment filters the water.

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 76/77
© 2012 Pearson Education, Inc.
Wastewater Treatment

8/22/2019 16. Mixtures
http://slidepdf.com/reader/full/16-mixtures 77/77
Wastewater Treatment