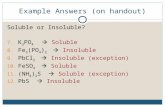
1 Selective Precipitation a solution containing several different cations can often be separated by...
-
Upload
francine-douglas -
Category
Documents
-
view
216 -
download
0
Transcript of 1 Selective Precipitation a solution containing several different cations can often be separated by...
1
Selective PrecipitationSelective Precipitation
a solution containing several different cations can often be separated by addition of a reagent that will form an insoluble salt with one of the ions, but not the others
a successful reagent can precipitate with more than one of the cations, as long as their Ksp values are
significantly different
a solution containing several different cations can often be separated by addition of a reagent that will form an insoluble salt with one of the ions, but not the others
a successful reagent can precipitate with more than one of the cations, as long as their Ksp values are
significantly different
2
What is the minimum [OH−] necessary to just begin to precipitate Mg2+ (with [0.059]) from seawater assuming
Ksp=2.06x10-13)?
What is the minimum [OH−] necessary to just begin to precipitate Mg2+ (with [0.059]) from seawater assuming
Ksp=2.06x10-13)?
precipitating may just occur when Q = Ksp
3
What is the [Mg2+] when Ca2+ (with [0.011]) just begins to precipitate from seawater?
What is the [Mg2+] when Ca2+ (with [0.011]) just begins to precipitate from seawater?
precipitating Mg2+ begins when [OH−] = 1.9 x 10-6 M
4
What is the [Mg2+] when Ca2+ (with [0.011]) just begins to precipitate from seawater?
What is the [Mg2+] when Ca2+ (with [0.011]) just begins to precipitate from seawater?
precipitating Mg2+ begins when [OH−] = 1.9 x 10-6 M
precipitating Ca2+ begins when [OH−] = 2.06 x 10-2 M
when Ca2+ just begins to precipitate out, the [Mg2+] has dropped from 0.059 M to 4.8 x 10-10 M
5
Qualitative AnalysisQualitative Analysis
an analytical scheme that utilizes selective precipitation to identify the ions present in a solution is called a qualitative analysis scheme wet chemistry
a sample containing several ions is subjected to the addition of several precipitating agents
addition of each reagent causes one of the ions present to precipitate out
an analytical scheme that utilizes selective precipitation to identify the ions present in a solution is called a qualitative analysis scheme wet chemistry
a sample containing several ions is subjected to the addition of several precipitating agents
addition of each reagent causes one of the ions present to precipitate out
13
Complex Ion FormationComplex Ion Formation
transition metals tend to be good Lewis acids
they often bond to one or more H2O molecules to form a hydrated ion H2O is the Lewis base, donating electron pairs to form
coordinate covalent bondsAg+(aq) + 2 H2O(l) [Ag(H2O)2
+](aq)
ions that form by combining a cation with several anions or neutral molecules are called complex ions e.g., Ag(H2O)2
+
the attached ions or molecules are called ligands e.g., H2O
transition metals tend to be good Lewis acids
they often bond to one or more H2O molecules to form a hydrated ion H2O is the Lewis base, donating electron pairs to form
coordinate covalent bondsAg+(aq) + 2 H2O(l) [Ag(H2O)2
+](aq)
ions that form by combining a cation with several anions or neutral molecules are called complex ions e.g., Ag(H2O)2
+
the attached ions or molecules are called ligands e.g., H2O
14
Complex Ion EquilibriaComplex Ion Equilibria
if a ligand is added to a solution that forms a stronger bond than the current ligand, it will replace the current ligand
Ag(H2O)2+
(aq) + 2 NH3(aq) Ag(NH3)2+
(aq) + 2 H2O(l)
generally H2O is not included, since its complex ion is always present in aqueous solution
Ag+(aq) + 2 NH3(aq) Ag(NH3)2
+(aq)
if a ligand is added to a solution that forms a stronger bond than the current ligand, it will replace the current ligand
Ag(H2O)2+
(aq) + 2 NH3(aq) Ag(NH3)2+
(aq) + 2 H2O(l)
generally H2O is not included, since its complex ion is always present in aqueous solution
Ag+(aq) + 2 NH3(aq) Ag(NH3)2
+(aq)
15
Formation ConstantFormation Constant
the reaction between an ion and ligands to form a complex ion is called a complex ion formation reaction
Ag+(aq) + 2 NH3(aq) Ag(NH3)2
+(aq)
the equilibrium constant for the formation reaction is called the formation constant, Kf
the reaction between an ion and ligands to form a complex ion is called a complex ion formation reaction
Ag+(aq) + 2 NH3(aq) Ag(NH3)2
+(aq)
the equilibrium constant for the formation reaction is called the formation constant, Kf
17
200.0 mL of 1.5 x 10-3 M Cu(NO3)2 is mixed with 250.0 mL of
0.20 M NH3. What is the [Cu2+] at equilibrium? 200.0 mL of 1.5 x 10-3 M Cu(NO3)2 is mixed with 250.0 mL of
0.20 M NH3. What is the [Cu2+] at equilibrium?
Write the formation reaction and Kf expression.Look up Kf value
Determine the concentration of ions in the diluted solutions
Cu2+(aq) + 4 NH3(aq) Cu(NH3)42+(aq)
18
200.0 mL of 1.5 x 10-3 M Cu(NO3)2 is mixed with 250.0 mL of
0.20 M NH3. What is the [Cu2+] at equilibrium? 200.0 mL of 1.5 x 10-3 M Cu(NO3)2 is mixed with 250.0 mL of
0.20 M NH3. What is the [Cu2+] at equilibrium?
Create an ICE table. Since Kf is large, assume all the Cu2+ is converted into complex ion, then the system returns to equilibrium
[Cu2+] [NH3] [Cu(NH3)22+]
Initial 6.7E-4 0.11 0
Change -≈6.7E-4 -4(6.7E-4) + 6.7E-4
Equilibrium x 0.11 6.7E-4
Cu2+(aq) + 4 NH3(aq) Cu(NH3)42+(aq)
19
200.0 mL of 1.5 x 10-3 M Cu(NO3)2 is mixed with 250.0 mL of
0.20 M NH3. What is the [Cu2+] at equilibrium? 200.0 mL of 1.5 x 10-3 M Cu(NO3)2 is mixed with 250.0 mL of
0.20 M NH3. What is the [Cu2+] at equilibrium?
Cu2+(aq) + 4 NH3(aq) Cu(NH3)22+(aq)Substitute in and
solve for x
confirm the “x is small” approximation
[Cu2+] [NH3] [Cu(NH3)22+]
Initial 6.7E-4 0.11 0
Change -≈6.7E-4 -4(6.7E-4) + 6.7E-4
Equilibrium x 0.11 6.7E-4
since 2.7 x 10-13 << 6.7 x 10-4, the approximation is valid
20
The Effect of Complex Ion Formation on Solubility
The Effect of Complex Ion Formation on Solubility
the solubility of an ionic compound that contains a metal cation that forms a complex ion increases in the presence of aqueous ligands
AgCl(s) Ag+(aq) + Cl−(aq) Ksp = 1.77 x 10-10
Ag+(aq) + 2 NH3(aq) Ag(NH3)2
+(aq) Kf = 1.7 x 107
adding NH3 to a solution in equilibrium with AgCl(s) increases the solubility of Ag+
the solubility of an ionic compound that contains a metal cation that forms a complex ion increases in the presence of aqueous ligands
AgCl(s) Ag+(aq) + Cl−(aq) Ksp = 1.77 x 10-10
Ag+(aq) + 2 NH3(aq) Ag(NH3)2
+(aq) Kf = 1.7 x 107
adding NH3 to a solution in equilibrium with AgCl(s) increases the solubility of Ag+
22
Solubility of Amphoteric Metal Hydroxides
Solubility of Amphoteric Metal Hydroxides
many metal hydroxides are insoluble eg Fe(OH)3, Al(OH)3, Co(OH)2
all metal hydroxides become more soluble in acidic solution shifting the equilibrium to the right by removing OH−
Fe(OH)3(s) [Fe(OH)2+](aq) + OH-(aq)
H3O+(aq)+OH-(aq) 2H2O(l)
Amphoteric metal hydroxides also become more soluble in basic solution acting as a Lewis base forming a complex ion
some cations that form amphoteric hydroxides include Al3+, Cr3+, Zn2+, Pb2+, and Sb2+
many metal hydroxides are insoluble eg Fe(OH)3, Al(OH)3, Co(OH)2
all metal hydroxides become more soluble in acidic solution shifting the equilibrium to the right by removing OH−
Fe(OH)3(s) [Fe(OH)2+](aq) + OH-(aq)
H3O+(aq)+OH-(aq) 2H2O(l)
Amphoteric metal hydroxides also become more soluble in basic solution acting as a Lewis base forming a complex ion
some cations that form amphoteric hydroxides include Al3+, Cr3+, Zn2+, Pb2+, and Sb2+
23
Al3+Al3+
Al3+ is hydrated in water to form an acidic solution
Al(H2O)63+
(aq) + H2O(l) Al(H2O)5(OH)2+(aq) + H3O+
(aq)
addition of OH− drives the equilibrium to the right and continues
to remove H from the molecules
Al(H2O)5(OH)2+(aq) + OH−
(aq) Al(H2O)4(OH)2+
(aq) + H2O (l)
Al(H2O)4(OH)2+
(aq) + OH−(aq) Al(H2O)3(OH)3(s) + H2O
(l)
Al3+ is hydrated in water to form an acidic solution
Al(H2O)63+
(aq) + H2O(l) Al(H2O)5(OH)2+(aq) + H3O+
(aq)
addition of OH− drives the equilibrium to the right and continues
to remove H from the molecules
Al(H2O)5(OH)2+(aq) + OH−
(aq) Al(H2O)4(OH)2+
(aq) + H2O (l)
Al(H2O)4(OH)2+
(aq) + OH−(aq) Al(H2O)3(OH)3(s) + H2O
(l)