1 Combining and Breaking Down Substances. 2 Compounds & Mixtures: What happens when you combine two...
-
Upload
kenneth-thomas -
Category
Documents
-
view
224 -
download
0
Transcript of 1 Combining and Breaking Down Substances. 2 Compounds & Mixtures: What happens when you combine two...

1
Combining and Breaking Down Substances

2
Compounds & Mixtures:
What happens when you combine two or more substances?
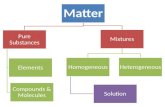
1. Compounds – is a substance that is made from two or more simple substances that can be broken down by chemical means.
* a compound always contains two or more elements joined in fixed proportions.
2. Mixture – is a physical combination of two or more substances.
* a mixture does not have a set number of elements joined in fixed proportions.

3
Types of mixtures: There are two types of mixtures:
1. Heterogeneous Mixtures – the parts of the mixture are noticeably different from one another.
ex. sand or gravel2. Homogeneous Mixtures – the substances are so evenly distributed that
is difficult to distinguish one from another.
* Solution – a mixture that forms when a substance dissolves (solute) in a liquid (solvent) and forms a homogeneous mixture.

4
A little review: Compounds are much more complex
than mixtures. We will have to review the structure of
an atom to understand how compounds work.

5
e-1st Period: Hydrogen (#1) Helium (#2)
nucleus
e-
e-
2nd Period: Lithium (#3) Neon (#10)
e- e-e- e-e- e- e-
e-
e-
e-e-
e-e-
1st energy level
2nd energy level
Electron Structures of Atoms

6
Elements in the same group have similar properties because they have same number of valence electrons (e- in outermost shell).
The number of valence electrons increases as you go from left to right across a period; there is no change going down a group.
*Ex: Alkali metals all have one valence electron: they all will form a white powder with cl.

7

8
Lewis structures of atomsLewis structure: shows only valence e- of an atom or
ion. Uses dots, representing e-, at top, bottom, right, and
left sidesEx: Carbon (6 e-) Fluorine (9 e-)
CValence
e-
Element symbol
F
e-
e-e-
e-
e-e-
e-
e-e-
e-
e-
e-e-
e-
e-

9
Lewis structures of first 20 elements
Most elements “want” a set of valence electrons like that of the chemically-stable noble gases, which have 8 valence electrons- (except He)

10
Chemical Bonds Chemical bond – force that holds atoms or
ions together Interaction occurs between valence electrons Examples: ionic, covalent
Chemical formula – shows the elements in a compound and the ratio of the atoms in the compound Example: formula for water is H20

11
Ionic Compounds When one or more e- are transferred from a
metal atom to a nonmetal atom, ions are formed.
Ionic bond: attractive force between oppositely charged ions
Na atom + :Cl: atom → Na +:Cl:sodium chloride
..:Cl:
....
. ...

12
Mg: atom + 2 :Cl: atom → Mg + 2 :Cl:magnesium chloride
.
.
.....

13
Covalent Compounds Covalent bonds occur in most “natural”
compounds like methane (CH4), ammonia (NH3), and large biological molecules (proteins, DNA, etc.)
Nonmetal atoms still “want” to have filled e- levels, but instead of transferring e- and forming ions, they share e-, forming a covalent bond. (“Co-valent” means “sharing valence”.)

14
Substances with covalent bonds exist as molecules (combinations of at least 2 nonmetal atoms)O
-C-O
*The bonds between C and O in carbon dioxide are covalent. CO2 exists as independent molecules.

15
*The simplest molecule: H2
The 1st energy levels of the H atoms
overlap.
The 2 e- are shared, and are
likely to be found anywhere
between the atoms.
Each H has 1 e-

16
H Cl
*Hydrogen chloride molecule, HCl If the elements are not the same, the bond is polar
covalent; the electrons are shared unequally.
Cl has a greater attraction than H for the 2 shared
e-
+Partial
positive charge -
Partial negative charge
Shared e- are closer to Cl,
making Cl slightly negative
:

17
Chemical Reactions Just as we can classify matter, we can classify
chemical reactions. Some of the general types of reactions follow:
1. Synthesis Reaction- is a reaction in which two or more substances react to form a single substance.
ex. Na + Cl NaClex. 2H2 + O2 2H2O
2. Decomposition Reaction- Opposite of a synthesis reaction. This a reaction in which a compound breaks down into two or more simpler substances.
ex. 2H2O 2H2 + O2

18
Chemical Reactions Cont.1. Replacement Reaction- is a reaction in which one or more elements take
the place of one or more elements in a compound, resulting in a new compound.
ex. Cu + 2AgNO3 2Ag + Cu(NO3)2

19

20
Chemical Reactions Cont.4. Combustion Reactions – is a reaction in which a substance reacts
rapidly with oxygen, which often produces heat and light.
ex. CH4 + 2O2 CO2 + 2H2O + heat & light

21
Factors that effect Reactions: Reaction rates depend on how often the particles collide. If the collisions occur more often the rate will increase
and vice versa. Factors that affect reaction rates include the following:
1. Temperature - Generally, an increase in temperature will increase a reaction rate.
ex. milk stored in a refrigerator -vs- on the counter
2. Surface Area – The more area exposed the faster the reaction will be.

22
Factors that effect Reactions Cont.
3. Stirring – Also increases the exposure of reactants to each other.
ex. washing machine 4. Concentration – The more reactants, the faster the particles will react.
ex. dye solution concentration 5. Catalysts – is a substance that affects the reaction rate without being
used up in the reaction.
* Used to speed up reactions or have a reaction occur at a lower temperature.
* Weakens the bonds holding substance together.