1 Chapter 6: pp. 103-116 Metabolism: Energy and Enzymes Copyright © The McGraw-Hill Companies, Inc....
-
Upload
elvin-wiggins -
Category
Documents
-
view
215 -
download
0
Transcript of 1 Chapter 6: pp. 103-116 Metabolism: Energy and Enzymes Copyright © The McGraw-Hill Companies, Inc....
1
Chapter 6: pp. 103-116
Metabolism: Energy and Enzymes
Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
solarenergy
heat
heat
heat
heat
Mechanical energy
Chemicalenergy
6.1 Cells and Energy Flow
What is energy?
Ability to do work
Allows LTs to carry out life process
All depend on solar energy
4
Flow of Energy
solarenergy
heat
heat
heat
heat
Mechanical energy
Chemicalenergy
Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
5
B. Laws of Thermodynamics
First law:
Law of conservation of energy
Energy cannot be created or destroyed, but
Energy CAN be changed from one form to another
6
Carbohydrate Metabolism
Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
carbohydrate muscle contraction
heat
Second law:
Law of entropy
When energy is changed from one form to another, there is a loss of usable energy
Waste energy goes to increase disorder
8
6.2 Metabolic Reactions and Energy Transformations
Metabolism:
Sum of cellular chemical reactions in cell
Free energy is the amount of energy available after a chemical reaction
FE of reactants – FE of products → If (-) then reaction occurs spontaneously
Endergonic ReactionsEndergonic Reactions Energy input requiredEnergy input required
Product has more free Product has more free
energy than starting energy than starting
substancessubstances
AnabolicAnabolic
Ex: protein synthesis, Ex: protein synthesis,
muscle contractionmuscle contraction
http://bp3.blogger.com/_6ZOUxSfmYeA/R19PgpZQIjI/AAAAAAAAABc/jlaGrT4-W3Y/s320/bio4.jpg
Exergonic ReactionsExergonic Reactions
Energy is releasedEnergy is released Products have Products have
less free energy less free energy than starting than starting substancesubstance
CatabolicCatabolic
http://www.emc.maricopa.edu/faculty/farabee/BIOBK/exergonic.gif
•In which direction is this reaction anabolic? •In which direction is the reaction catabolic?•Where is the energy of the ATP molecule stored?
12
A. ATP and Coupled Reactions
Adenosine triphosphate (ATP)High energy compound used to drive metabolic reactionsConstantly being generated
Composed of:Adenine and ribose (together = adenosine), andThree high energy phosphate groups
AdvantagesCommon and universalLittle wasteBreakdown coupled with endergonic
Coupled Reactions
Rxns occur in same time and place so exergonic drives endergonic
Usually exergonic = ATP hydrolysis because more energy released than consumed
2 ways to couple ATP hydrolysisATP used to energize reactant (Cell. Resp)ATP used to change shape of reactant ( muscle contraction)
Drives energetically unfavorable reactions to create order for life
16
Work-Related Functions of ATP
Primarily to perform cellular work
Chemical Work - Energy needed to synthesize macromolecules
Transport Work - Energy needed to pump substances across plasma membrane
Mechanical Work - Energy needed to contract muscles, beat flagella, etc
17
6.3 Metabolic Pathways
Reactions are usually occur in a sequence
Products of an earlier reaction become reactants of a later reaction
Such linked reactions form a metabolic pathway
Begins with a particular reactant,
Proceeds through several intermediates, and
Terminates with a particular end product
AB C D E FG
“A” is InitialReactant
“G” is EndProduct
B, C, D, E, and Fare Intermediates
Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer.
19
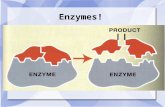
Enzymes
Enzymes
Protein molecules that function as catalysts
The reactants are called substrates
Regulate by eliminating side reactions
Each reaction in a metabolic pathway requires a unique and specific enzyme
End product will not appear unless ALL enzymes present and functional
E1 E2 E3 E4 E5 E6A B C D E F G
Four Features of EnzymesFour Features of Enzymes1) Enzymes do not make anything happen that could 1) Enzymes do not make anything happen that could
not happen on its own. They just make it happen not happen on its own. They just make it happen much faster.much faster.
2) Reactions do not alter or use up enzyme molecules.2) Reactions do not alter or use up enzyme molecules.
3) The same enzyme usually works for both the 3) The same enzyme usually works for both the forward and reverse reactions.forward and reverse reactions.
4) Each type of enzyme recognizes and binds to only certain substrates.
Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer.
23
A. Energy of Activation
Energy must be added to at least one reactant to initiate the reaction
Enzyme Operation:
Enzymes operate by lowering the energy of activation
Bring the substrates into contact with one another
Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer.
25
B. Enzyme-Substrate Complex
Substrate = reactants
Active site = small part that binds substrate specifically
Induced fit model – active site changes slightly for optimum fit
Sometimes they participate Ex: Trypsin protein digestion
Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer.
30
C. Factors Affecting Enzyme Activity
Substrate concentrationIncrease substrate increase collisions until a max rateAmount of enzyme can also limit
TemperatureEnzyme activity increases with temperatureWarmer temperatures cause more effective collisions between enzyme and substrateHowever, hot temperatures destroy enzyme
Optimal pH
Normal configurations
Globular structure dependent on HB between R groups
Change pH will change ionization of side chains
Extreme change will cause inactivity
Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer.
Temperature
Increase temp, increase activity because of better collisions
Will level out and then decline because of changed shape
Exceptions: prokaryotes in hot springs
Siamese coat color
34
Enzyme CoFactors
Molecules required to activate enzymeInorganic = Cu, Zn, Fe
Coenzymes are organic cofactors
Vitamins become part of conenzymes
Phosphorylation – some require addition of a phosphate
Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer.
Specific Enzyme Inhibition
IrreversibleForms strong
covalent bonds at active site
React with -SH, -OH, or acid groups
Ex: Ag, Hg, Pb
NoncompetitiveReversible
Changes shape, thus changing active site
37
Irreversible Inhibition
Materials that irreversibly inhibit an enzyme are known as poisons
Cyanides inhibit enzymes resulting in all ATP production
Penicillin inhibits an enzyme unique to certain bacteria
Heavy metals irreversibly bind with many enzymes
Nerve gas irreversibly inhibits enzymes required by nervous system
Competitive
Inhibitor and substrate both go after active site
Example:Ethanol to
acetaldehyde to acetic acid
2nd rxn fast but Anatabuse block causing side effects
Stop drinking
Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer.
What molecule does grass make available to an herbivore?
What happens to this molecule during cellular respiration?
Carbon dioxide is _____ to produce glucose during PS and glucose is ____ to produce ATP during CR.
6.4 Organelles and Energy Flow
PhotosynthesisChloroplast capture solar
energy
Convert using electron transport chain to ATP
Used to reduce CO2 to glucose
Cellular RespirationH atoms from C are bonded
to O2
Mitochondria breaks down carbs to produce ATP by an electron transport chain
45
Electron Transport Chain (ETC)
Membrane-bound carrier proteins that pass e- from one to another
Physically arranged in an ordered series
Starts with high-energy e- and low-energy e- leave
Each carrier is reduced and then oxidized as e- are transferred
46
A Metaphor for the Electron Transport Chain
Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
high-energyelectrons
low-energyelectrons
electrontransport chain
ATP
energy forsynthesis of
e
e
ETCs
a. are found in both mitochondria and chloroplasts
b. release energy as e- are transferred
c. are involved in ATP production
d. are located in a membrane
e. all of the above
ATP Production
• Chemosmosis
• Carriers found on thylakoid membrane of chloroplast and cristae of mitochondria
• H+ collects on one side of the membrane
• Establishes an e- gradient
• ATP Synthase complexes
51
Chemiosmosis
Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
ATPsynthasecomplex
energy fromelectron transfers
H+ H+
High H + concentration
Low H + concentration
H + pump in electrontransport chain
H+
H+
H+
H+ H+
ATP
ADP + P
Chemiosmosis is dependent on
a. the diffusion of water across a differentially permeable membrane
b. an outside supply of phosphate and other chemicals
c. the establishment of an electrochemical H+ gradient