01 - Publication - A use friendly Mutliscale lung modeling suite and applications
-
Upload
viraj-shah -
Category
Documents
-
view
15 -
download
0
Transcript of 01 - Publication - A use friendly Mutliscale lung modeling suite and applications

A User Friendly Multiscale Lung Modeling Suite and Application
A Thesis
Submitted to the Faculty
of
Drexel University
by
Viraj Nignesh Leena Shah
in partial fulfillment of the
requirements for the degree
of
Master of Science in Biomedical Engineering
December 2007


Multiscale Lung Modeling ii Shah Viraj
Dedications
First I shall like to thank my parents Mr. Nignesh and Mrs. Leena Shah from the bottom
of my heart for their love, affection and guidance for the pursuit of my studies at Drexel
University.
I shall take this opportunity to thank my brother Naishal and the rest of my family for
always being there for me.
Next I shall like to thank my friends Smiral, Shivani, Abi, Heman, Ashish, Nirali and all
my other friends at Drexel and from India for making me a better person each day with
their suggestions and guidance. Finally I shall pay homage to my Grandmother’s and my
brother Jay who always showered their blessings from wherever they are.

Multiscale Lung Modeling iii Shah Viraj
Acknowledgements
I would like to thank my Thesis advisor Dr. Andres Kriete for his outstanding guidance
and vision throughout this accomplishment and without whom this Project would not had
been possible. I shall also like to thank Saloni Shah for being an associate for the setup of
this Project and the previous collaborators Thorsten Denhard and Andreas Schmidt who
were the pioneers and developers of the foundations of this software. I shall also look
forward to Udaykiran Thummalapalli for continuing this project. Lastly I shall thank my
committee members, Dr Rami Seliktar, Dr. Fred Allen and Dr. Todd Doehring to share
their insights about the Project.

Multiscale Lung Modeling iv Shah Viraj
Table of Contents Dedications ......................................................................................................................... ii
Acknowledgements............................................................................................................ iii
Table of Contents............................................................................................................... iv
Lists of Tables.................................................................................................................... vi
List of Figures ................................................................................................................... vii
Abstract .............................................................................................................................. xi
Chapter 1. ........................................................................... 1 Background and literature
1.1. .............................................................................. 1 The anatomy of human lungs
1.2. .............................................................................. 8 Lung volumes and capacities
1.3. ................................................................................... 10 Mechanics of respiration
1.4. .................. 11 Structural change in the respiratory part of the lung (emphysema)
1.5. ..................................................... 16 Surface to volume (SV_Ratio) ratio change
Chapter 2. ..................................... 19 Previous computational models and assumptions
2.1. ....................................................................................................... 20 Introduction
2.2. ................................................................................................ 21 Previous models
2.2.1. ........................................................................ 22 Computer reference model
2.2.2. ............................................................................. 23 Modeling gas transport
2.2.4. ............................................................. 31 Benefits of QT for lung modeling
2.3. .............................................. 32 Design of user interface and steps in simulation

Multiscale Lung Modeling v Shah Viraj
2.4. ................................................................. 39 Example of modeling a normal lung
2.7 ....................................................................................................... 40 Emphysema
2.5. .................... 41 Measurement of histological and micro CT on lung emphysema
2.6. ............................................ 43 Extension of user interface to handle emphysema
Chapter 3. ....................................................................................................... 45 Results
3.1. ......................................... 45 Results of gas transport simulation in normal Lung
3.2. ....................................................................... 46 Results for emphysematic lungs
3.2.1. .......................................... 51 Emphysematic data with change in SV_Ratio
Chapter 4. ................................................................................................. 59 Discussion
4.1. ........................................................................ 61 Future extensions of the model:
List of References ............................................................................................................. 62
Vita.................................................................................................................................... 68

Multiscale Lung Modeling vi Shah Viraj
Lists of Tables Table 1: Parameters for the j airway segmentth .......................................................................24
Table 2: Quantitative analysis of the lung (Coxson, Rogers et al. 1999) ...............................41

Multiscale Lung Modeling vii Shah Viraj
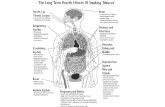
List of Figures Figure 1: A) Lung anatomy, B) External anatomy of lungs (Machen 2007)............................2
Figure 2: A) Surface area of airway branching, B) Structure of a lung lobule (Machen
2007) ................................................................................................................................................3
Figure 3: Tree classification using different orderings (a) Weibel generations, (b) Strahler
Ordering and (c) Horsfield ordering (Tawhai 2001) ...................................................................4
Figure 4: Hierarchy of airways in human lung (Tawhai 2001) ...................................................5
Figure 5: A) Microscopic alveoli structure (Machen 2007), B) Cross section of Alveoli
(Greer 2007)....................................................................................................................................7
Figure 6:Lung volumes and capacities, TLC –Total lung capacity, VC – Vital capacity, IRV
– Inspiratory reserve volume, ERV – Expiratory reserve volume, RV – Residual volume, FRC
– Functional residual capacity, TV – Tidal volume (Nosek 2007)............................................10
Figure 7: Types of airflow (Wilmot C Ball Jr. 1996).....................................................................11
Figure 8: Types of emphysema; A) Normal acinus; B) Centrilobular emphysema C)
Panacinar emphysema; D) Paraseptal emphysema; E) Irregular emphysema (Roy 2007)
.......................................................................................................................................................14
Figure 9: Air trappings in Expiration due to emphysema A) Normal expiration; B) Impaired
expiration due to decreased elastic recoil (Nosek 2007).......................................................16
Figure 10: A) terminal airspaces composed of hemispheres; B) Airspaces dilate
concentrically shown with 2 positions; C) One hemisphere joins with another to form a
spherule; D) Circumferential segment of tissue with known diameter and length (L)
(Carton, Clark et al. 1964) ..........................................................................................................19
A model lung can be visualized as consisting of a large number of communicating
spaces of equal volume which are the alveoli. Assume that the spaces are hemispheres,
(Figure 10) which dilates equally and concentrically under a distending pressure, and
that their expansion is opposed only by fibers circumferentially distributed in their walls.

Multiscale Lung Modeling viii Shah Viraj
Two hemispheres can be grouped together as one spherule (Figure 11). The work of
expansion per hemisphere is half that of the spherule. The restraining fibers lie along
segments of great circles. To calculate work done on expansion, these can be grouped
together as a circumferential band or a segment (L) of known diameter..........................19
Figure 12: An airway bifurcation used in modeling gas concentration and uptake in
airways. Airway segments s1 and s2 are daughter segments of j; C is the concentration in
the segment; L is the length of the segment; A is the cross sectional area at the entrance
of the segment(Mercer, Anjilvel et al. 1991) ............................................................................26
Figure 13: Conical segments used to model a ventilatory unit. Gas flow from parent
airway generation is represented by leftmost segment. Alveolar ducts correspond to
darkened region of each conical segment where A - alveolar duct cross-sectional
area; A - alveolar plus alveolar duct cross-sectional area; L - length of each conical
segment. Ventilation-induced changes in ventilatory unit size are followed by increase in
length and cross-sectional area of conical segments.(Mercer, Anjilvel et al. 1991)
Dj
j
..........27
Figure 14: Flowchart of the simulation.......................................................................................32
Figure 15: Top) Graphical User Interface (GUI) for loading a tree geometry using the
software Bottom) Graphical User Interface (GUI) for opening a data file using the
software ........................................................................................................................................34
Figure 16: A tree structure of the lung loaded using the software and the path of gas flow
on the tree structure highlighted using the color blue............................................................35
Figure 17: A) Describes the lung physiology parameters; B) Describes the respiratory unit
(acinus geometry) .......................................................................................................................36
Figure 18: Display of approximate duration of simulation after the process is initiated .....37
Figure 19: Left) Open the result file; Right) Select the output file to open............................38
Figure 20: Left) Write the results; Right) Results data analysis time duration and acini data
selection option ...........................................................................................................................39

Multiscale Lung Modeling ix Shah Viraj
Figure 21: Log file of the simulation with the model parameters and duration of simulation
.......................................................................................................................................................40
Figure 22: 1) Micro CT of normal lung; 2) Histogram of figure 1; 3) Contrast stretched figure
2; 4) Thresholded figure 3............................................................................................................42
Figure 23: 1) Micro CT of Centrilobular emphysema; 2) Thresholded figure 1; 3) Results of
the SV_Ratio for figure 2..............................................................................................................43
Figure 24: Change in SV_Ratio to handle emphysema with other parameters on the GUI
interface .......................................................................................................................................44
Figure 25: A) Concentration (%) to time (sec) for a healthy lung with a mean +/- std. dev
of 3.753+/- 6.10; B) Concentration (%) to time (sec) for an Emphysematic lung with a
mean +/- std. dev of 2.87 +/-5.99; C) Difference in Concentration (%) to time (sec) from a
healthy to an emphysematic lung with a mean +/- std. dev of 0.883+/-1.40 .....................45
Figure 26: A) Surface to volume ratio to disease conditions of emphysema (Coxson,
Rogers et al. 1999) .......................................................................................................................46
Figure 27: 1, 3) Difference of each repetition with the first repetition showing the
repeatability of software output; 2, 4) Change in concentration for 7 generation of acini
with a mean +/- std. of 0.81+/- 0.44...........................................................................................47
Figure 28: 1, 3) Difference of each repetition with the first repetition showing the
repeatability of software output; 2, 4) Change in concentration for 7 generation of acini
with a mean +/- std. of 0.87 +/- 0.46..........................................................................................48
Figure 29: Difference in normal lung (T_V is 500 mL and S_V ratio is 34.6 mm ) to
emphysematic lung (T_V is 250 mL and S_V ratio is16.5 mm ) concentration with 1.4 +/-
0.95 (mean +/- std. dev.)
-1
-1
............................................................................................................49
Figure 30: Difference in normal lung (T_V is 500 mL and S_V ratio is 34.6 mm ) to
emphysematic lung (T_V is 500 mL and S_V ratio is16.5 mm ) concentration 0.757 +/-0.79
(mean +/- std. dev.)
-1
-1
....................................................................................................................50

Multiscale Lung Modeling x Shah Viraj
Figure 31: Normal lung uptake with a men +/- std. dev. of 2.57 +/- 0.11 for acini segment
98 and 7 generations of acini for 20 breathing cycles (4sec *20 = 80 sec)..........................51
Figure 32: Uptake for mild emphysema disease condition with a mean +/- std. dev. of
1.55 +/- 0.05 for acini segment 98 and 7 generations of acini for 20 breathing cycles (4sec
*20 = 80 sec) .................................................................................................................................52
Figure 33: Severe emphysema uptake with a mean +/- std. dev. of 0.48 +/- 0.01 for acini
segment 98 and 7 generations of acini for 20 breathing cycles (4sec *20 = 80 sec) .........53
Figure 34: Left to right, normal lung (12.08 +/- 0.28), mild emphysema (13.81 +/- 0.24) and
severe emphysema (15.39 +/-0.21), acini segment 98 and 7 generations of acini for the
20 breathing cycleth ....................................................................................................................54
Figure 35: Difference in the normal lung to mild emphysema of 1.74 +/- 0.06 for 20
breathing cycle of 4 sec (The negative sign is due to mild emphysema being greater in
concentration compared to normal lung)
th
..............................................................................55
Figure 36: Difference in normal lung to severe emphysema of 3.31 +/- 0.12 for 20
breathing cycle of 4 sec (The negative sign is due to severe emphysema being greater in
concentration compared to normal lung)
th
..............................................................................56
Figure 37: Concentration ratios for 1) Normal to mild 1 breathing cycle with a mean +/-
std of 1.2 +/- 0.3; 2) Normal to mild for 20 breathing cycle with a mean +/- std of 0.87 +/-
0.005; 3) Normal to mild for 1 breathing cycle with a mean +/- std of 1.833 +/- 1.88; 4)
Normal to mild for 20 breathing cycle with a mean +/- std of 0.78 +/- 0.009
st
th
st
th ....................57

Multiscale Lung Modeling xi Shah Viraj
Abstract A User Friendly Multiscale Lung Modeling Suite and Application
Viraj Shah Andres Kriete, Ph.D.
The development of computational lung models and the functional simulation of gas
transport is a promising avenue to investigate the effects of structural alterations as they
relate to disease and aging on the lung function. Since many changes such as emphysema
originate from the respiratory units (acini), which are the end units of the bronchial tree,
predictions about the impact on the lung function on this level of organization critically
rely on a precise modeling of the entire lung. The computational modeling suite, which
extends previous versions by a user friendly interface based on QT, allows modifying
structural properties and executing functional simulations of the entire lung including the
respiratory units. Application of this utility is demonstrated for emphysema, a disease and
aging related condition with an abnormal permanent enlargement of air spaces with the
destruction of cell walls leading to decrease in the alveolar surface area to volume ratio.
The results demonstrate the predictability of loss in function such as oxygen uptake and
change in gas concentration profiles over the breathing cycle with the severity of the
disease.


Multiscale Lung Modeling 1 Viraj Shah
Chapter 1. Background and literature
1.1. The anatomy of human lungs
The lungs are respiratory organs which are marvelously efficient and beautifully
constructed. The ventilating passages are compact and symmetrical, but there is no
conformity with regards to the finer branches and ramifications at the level of acini
(respiratory units) and alveoli and about their physiological impact on aging and disease
conditions. The development of computational lung models and the functional simulation
of gas transport is a promising avenue to investigate the effect of structural changes on
the lung function. Since acini constitute the end units of the bronchial tree, predictions
about structural changes on this level of organization critically rely on a precise modeling
of the entire lung. Therefore we first describe the structural hierarchy of the lung, which
is considered in the computational model and subsequently an approach to represent the
respiratory units in this model, suitable for simulation. While the essential steps for the
simulation process and the structural analysis of human lungs have been published
elsewhere (Mercer and Crapo 1987; Kriete 1998; Schmidt, Zidowitz et al. 2004), here we
extend the software with a user friendly interface, that allows to run simulations and
modify structural properties related to disease and aging.
As shown in figure 1 the respiratory system consists of the upper respiratory system
(mouth, nasal cavity, pharynx, and larynx) and the lower respiratory system (trachea,
bronchi, and lungs). The lower respiratory system is enclosed in the thorax, bounded by
ribs, spine and diaphragm. Each lung is conical in shape with its base resting on the
diaphragm and its apex extending superiorly to a point approximately 2.5 cm superior to

Multiscale Lung Modeling 2 Viraj Shah
each clavicle. Lungs have a tree like structure where the trachea is the stem and there are
bronchioles, ducts and sacs which divide using bifurcation or trifurcation.
Figure 1: A) Lung anatomy, B) External anatomy of lungs (Machen 2007)
The trachea branches into two lobar bronchi, one to each lung. These primary bronchi
divide into secondary bronchi as they enter their respective lungs. The secondary
bronchi, two to the left lung and three to the right lung, conduct air to each lung.
Secondary bronchi give rise to tertiary bronchi which extend to the lobules. There are
nine lobules in the left lung and ten lobules in the right lung (Greer 2007). Bronchioles
divide numerous times to become terminal bronchioles which then divide into respiratory
bronchioles. As show in figure 2 the internal airway diameter falls from 500 µm to 270
µm between acinar generations 0 and 10, whereas the outer diameter remains constant at
700 µm. Each respiratory bronchiole branches approximately 22 more times to form
alveolar ducts that end as clusters of air sacs called alveoli. Alveolar sac is composed of

Multiscale Lung Modeling 3 Viraj Shah
two or more alveoli that share a common opening. Each cluster of alveoli is surrounded
by elastic fibers and a network of capillaries.
Figure 2: A) Surface area of airway branching, B) Structure of a lung lobule (Machen 2007)
A terminal bronchiole is a bronchiole at the end of the conducting zone. Alveoli surface
at the transition into the respiratory zone. When the parent divides into two it is a
bifurcation or dichotomy; division into three daughter branches is a trifurcation. Different
ordering schemes have been developed to define the branching hierarchy; an overview is
given by the following methods:

Multiscale Lung Modeling 4 Viraj Shah
Figure 3: Tree classification using different orderings (a) Weibel generations, (b) Strahler Ordering and (c) Horsfield ordering (Tawhai 2001)
Weibel Generations:
As shown in figure 3a the positions are defined in relation to the stem branch which is the
trachea. It continues towards the peripheral branches. The trachea has the lowest
generation number and each subsequent branch division produces daughter branches
which are one generation higher than the parent branch.
Strahler Ordering:
It was originally used on river systems. As shown in figure 3b it begins at the terminal
bronchiole which is usually 1. In the first part it classifies the parent branch as one order
higher than the two daughter branches of the same order or as the same order as the
highest ordered daughter branch. The second stage replaces the parent and daughter
branches of the same order with a branch of the same order.
Horsfield Ordering:
As shown in figure 3b it is an ordering system based on Strahler ordering which is more
appropriate for a lung tree. It assigns the lowest order to the terminal branches and the

Multiscale Lung Modeling 5 Viraj Shah
parent branches are one order higher than the daughter branch of the highest order. It
produces more branches and orders than Strahler ordering for asymmetrical trees. It has
the number of orders same as the number of Weibel generations. It is better than Weibel
generations in grouping functionally similar branches in asymmetrical trees. Horsfield et
al. (Horsfield and Cumming 1968) developed the concept of delta which is the difference
in Horsfield order of two daughter branches that arise from the same parent branch. A
tree with delta = 0 is symmetric, delta = 1 describes a tree with regular asymmetry of one
order difference between daughters and higher delta values describe more asymmetrical
trees.
Figure 4: Hierarchy of airways in human lung (Tawhai 2001)
As shown in figure 4 the entrance to the exchange zone of gases begins at the respiratory
bronchioles, which is after the conducting airway zone. They are distal to the terminal

Multiscale Lung Modeling 6 Viraj Shah
bronchioles and continue into alveolar ducts which are distal to the respiratory
bronchioles.
Alveolar ducts are the bronchioles which have alveoli on their walls in which the process
of respiration takes place. Alveolar ducts are densely studded with alveoli which form the
major part of the terminal air passages (Schulze 1871).
Approximately 40 % of the alveoli of the acinus are located on respiratory bronchioles,
including alveolar ducts, whereas 60 % are on the alveolar sacs. A similar ratio exists
between the volumes of the respiratory branches and the alveolar sacs. The size of alveoli
increases and clusters of alveoli become more numerous at the periphery. The pulmonary
alveolus comprises complex alveolated airways connected to a first order respiratory or
transitional bronchiole wherein all airways participate in gas exchange, whereas all
airways proximal to the transitional bronchiole are purely of a conducting nature. Alveoli
are functionally the largest unit in which all airways participate in gas exchange. Bulk of
acinar air moves along the intracinar airways mostly by molecular diffusion into the
residual air, whereas some oxygen diffuses radially into the alveoli.
Each alveolus is about 0.1 or 0.2 mm in diameter and each is surrounded by capillaries.
Gases are exchanged between the air and the blood by diffusion. A pair of human lungs
has about 300 million alveoli - (Dunnil 1962), providing a respiratory surface of about 70
m2 (Weibel 1963). The part of the airway that participates in gas exchange with the
pulmonary capillary blood consists of the respiratory bronchioles, alveolar ducts, and the
alveoli themselves.
Acini are complex alveolated airways distal to the terminal bronchioles, beginning with
the first order respiratory or transitional bronchiole. The longitudinal path length of

Multiscale Lung Modeling 7 Viraj Shah
acinar airways which is defined as the distance along the ducts from the transitional
bronchiole to the alveolar sacs is on an average 8.8 mm. To model the O2 transfer from
the transitional bronchiole to the alveolar sacs the branching pattern, airway diameters
and the total length of the pathway from the entrance into the acinus to the terminal sacs,
as well as its variation between different pathways within the tree is required - (Haefeli-
Bleuer and Weibel 1988).
Figure 5: A) Microscopic alveoli structure (Machen 2007), B) Cross section of Alveoli (Greer 2007)
The acinus geometry is divided into number of generations (n), length of segment of each
acinus generation (L), acinus duct area (Ad) for each individual acinus generation and the
cross section of each acinus generation (A). The acinus includes the terminal bronchiole
and all other branches of airways (respiratory bronchioles) and air spaces (alveolar ducts,
alveolar sacs, and alveoli) distal to the terminal bronchiole. A branch is an airway or air
space with a single axial direction. A terminal bronchiole (TB) is an airway immediately
proximal to the respiratory bronchiole; its walls are covered with bronchial epithelium. A

Multiscale Lung Modeling 8 Viraj Shah
respiratory bronchiole (RB) is an airway with RB or ducts as major outlets; its walls have
a significant amount (> 5 %) of bronchial epithelium and a few alveoli and/or sacs. A
duct (alveolar duct or AD) is an air space with several outlets: one or more sacs or ducts
and one or more alveoli. Except for limited (< 5 %) patches of bronchial epithelium, its
walls consist entirely of the margins of orifices of its single inlet and several outlets. It is
not an air space with only alveoli as outlets. A sac (alveolar sac or AS) is an air space
with only alveoli as outlets. An alveolus (A) is a terminal air space without outlets. If a
terminal air space has low partitions (i.e., mean partition height is 10 % or less than mean
orifice diameter), it is defined as a sac with daughter alveoli. An alternate definition
would be to call this terminal airspace a complex alveolus. In numbering generations, the
number increases as one move’s distally. Siblings belong to the same generation;
daughters and progeny are distal; parents are proximal. One can begin counting
generations at several different sites in the lung, e.g., at the start of any new type of
airway or air space. Thus the same duct might be identified as a 22nd generation branch
of the pulmonary tree, a 7th generation branch of the acinus, and a 3rd generation branch
of ducts.
1.2. Lung volumes and capacities
As shown in figure 8 the air in the lung is classified in two divisions, which are the lung
volumes which can be breathed by the subjects and the lung capacities which include two
or more primary volumes.
Tidal volume is the volume of air inhaled and exhaled with each breath and it is 500
(milliliter) mL in a resting human. It signifies the normal depth of breathing.

Multiscale Lung Modeling 9 Viraj Shah
Inspiratory reserve volume is the additional amount of air which can be inspired at the
end of normal inspiration and 3300 mL is a normal value.
Expiratory reserve volume is the amount of air that can be expired out forcefully after
normal expiration and 1000 mL is the normal value.
Residual volume is the air that remains in the lungs even after the forced expiration and it
helps to aerate the blood in between breathing and expiration and 1200 mL is the normal
value.
Inspiratory capacity is the maximum volume of air that can be inspired starting from end
expiratory position. It includes tidal volume and inspiratory reserve volume and 3800 mL
is the normal value.
Vital capacity is the maximum amount of air which can be expelled out forcefully after a
maximal inspiration. It includes the inspiratory reserve volume, tidal volume and
expiratory reserve volume and 4800 mL is the normal value.
Functional residual capacity is the volume of air remaining in the lungs after normal
expiration. It includes expiratory reserve volume and residual volume, and 2200 mL is
the normal value.
Total lung capacity is the amount of air present in the lungs after a maximal inspiration
and it includes all the volumes and 6000 mL is the normal value.

Multiscale Lung Modeling 10 Viraj Shah
Figure 6:Lung volumes and capacities, TLC –Total lung capacity, VC – Vital capacity, IRV – Inspiratory reserve volume, ERV – Expiratory reserve volume, RV – Residual volume, FRC – Functional residual capacity, TV – Tidal volume (Nosek 2007)
1.3. Mechanics of respiration
Air flows into and out of the lungs when air pressure within the lung differs from the
pressure of external air. As shown in figure 7 when alveolar pressure is less than
atmospheric pressure, air flows into the lungs, and inspiration occurs. The pressure in the
lungs is varied by changes in the volume of the thoracic cavity. These changes are
brought about by the contraction and relaxation of the muscular diaphragm and the
intercostal muscles. Inhalation is accomplished by contracting the diaphragm, which
flattens it and lengthens the thoracic cavity and by contracting the intercostal muscles that
pull the rib cages up and out. These movements enlarge the thoracic cavity, the pressure

Multiscale Lung Modeling 11 Viraj Shah
within it falls, and air moves into the lungs. Air is forced out of the lungs as the muscles
relax, reducing the volume of the chest cavity and increasing the pressure.
Figure 7: Types of airflow (Wilmot C Ball Jr. 1996)
During quiet breathing, laminar flow exists from the medium-sized bronchi down to the
level of the bronchioles. During exercise, when the air flow is more rapid, laminar flow
may be confined to the smallest airways. Transitional flow, which has some of the
characteristics of both laminar and turbulent flow, is found between the two along the rest
of the bronchial tree.
1.4. Structural change in the respiratory part of the lung (emphysema)
Emphysema is a condition of the lung characterized by abnormal, permanent enlargement
of the air spaces distal to the terminal bronchiole, accompanied by destruction of their
walls (ATS 1962). Similar definitions have been adopted by the World Health
Organization (WHO 1961). Destruction of emphysema is classified with parameters of
fenestrae (the presence of abnormal holes), the destructive index, the loss of alveolar

Multiscale Lung Modeling 12 Viraj Shah
surface area, mean linear intercept (Lm), air space wall per unit volume (AWUV) and
loss of alveolar attachments (Thurlbeck and Muller 1994).
Fenestrae are discontinuities of alveolar walls seen on thick sections of human lungs
(Boren 1962). These structural alterations can be diagnosed histopathologically or by
using micro – CT imaging techniques (Watz, Breithecker et al. 2005)
The destructive index (DI) is a recent innovation (Snider) suggested as a criterion for
alveolar wall destruction. The DI has three components:
1) Breaks in alveolar walls (DIb)
2) Type II cell metaplasia of alveolar walls, often accompanied by some
alveolar wall fibrosis (DIf)
3) Classic emphysema (DIe)
Loss of alveolar surface area is a good method of measuring destruction. Lm is the length
of a test line placed over histologic slides of the lung divided by the number of times the
line crosses alveolar walls (not surfaces). It is an approximation of air space (alveolar
ducts, alveolar sacs, and alveoli) size; it is not the mean chord length of alveoli. Alveolar
surface area (SA) is calculated from (Weibel 1963; Underwood 1970).
SA = 4V / Lm …Equation 1
In this formula, V is the volume of the lung in which Lm is measured. Rearrangement of
this formula shows that SV_Ratio (AWUV) is 4/Lm. Lm and AWUV (Gillooly and
Lamb 1993; Gillooly and Lamb 1993)) are thus reciprocals. Lm will increase if alveolar
walls are destroyed, because their loss results in fewer intercepts, or if there is over
inflation, which causes a greater distance between intercepts.

Multiscale Lung Modeling 13 Viraj Shah
Loss of alveolar attachments is considered to be a cause of airflow obstruction in
emphysema (Pratt, Haque et al. 1961; Anderson and Foraker 1962), especially on
expiration, loss of alveolar attachments to bronchioles has recently been described as
representing “an early stage of the destruction of lung parenchyma”(Saetta, Ghezzo et al.
1985). Bronchioles are thought to be tethered by their attachment to alveolar walls, and
associations have been described between loss of alveolar wall attachments and the
percentage of alveoli destroyed (Wright 1989) or the presence and severity of
macroscopic emphysema (Petty, Silvers et al. 1986; Nagai A 1991). There is progressive
destruction of alveoli and the surrounding tissue that supports the alveoli. With more
advanced disease, large air cysts develop where normal lung tissue used to be. Air is
trapped in the lungs due to lack of supportive tissue which decreases oxygenation.
Destruction may be recognized by the disorder of the respiratory airspaces. The major
mechanism of airflow limitation is loss of elastic recoil.
Primary emphysema:
It is commonly linked to an inherited deficiency of the enzyme α1 – antitrypsin which is
a major component of α1 – globulin which is a plasma protein and as seen from figure 5.
It inhibits the action of many proteolytic enzymes and so individuals with the deficiency
of this enzyme have a greater likelihood of developing this disease.
Secondary emphysema:
It is caused due to the inability of the body to inhibit proteolytic enzymes in the lungs due
to cigaratte smoke and air pollution.

Multiscale Lung Modeling 14 Viraj Shah
A
D B
C E
Figure 8: Types of emphysema; A) Normal acinus; B) Centrilobular emphysema C) Panacinar emphysema; D) Paraseptal emphysema; E) Irregular emphysema (Roy 2007) Types of emphysema classified according to the portion of the acinus first involved with the disease (University 2005)
1.) Centrilobular (Centriacinar) emphysema: As seen from figure 8b there is
septal destruction in the respiratory bronchioles and alveolar ducts usually in the
upper lobes of the lung. The alveolar sac which is distal to the repiratory
bronchiole remains intact. It occurs mainly in smokers with chronic bronchitis. It
involves the proximal part of the acinus. The destroyed and enlarged respiratory
bronchioles form airspaces that are separated from each other and from lobular

Multiscale Lung Modeling 15 Viraj Shah
septa by normal alveolar ducts and sacs. It is most common and most severe in the
upper zones of the lung (upper lobe and superior segment of lower lobe).
2.) Panacinar (panlobular) emphysema: As seen from figure 8c it involves damage
in the entire acinus which is more randomly distributed and involves lower lobes
of the lungs. It occurs in patients with α1 – antitrypsin deficiency. Panacinar
emphysema is often associated with centrilobular emphysema where the
panacinar form tends to occur in the lower zones of the lung and centrilobular
emphysema is seen in the upper ones.
3.) Paraseptal (subpleural) emphysema: As seen from figure 8d it involves the
alveolar ducts and sacs at the emphysematous areas. It is seen typically in a young
adult with history of a spontaneous pneumothorax and is also seen in older
patients with centrilobular emphysema.
4.) Irregular emphysema: As seen from figure 8e there is no consistent relationship
to any alveoli, bronchioles and ducts but is always associated with fibrosis.
Effects of emphysema:
1.) As seen from figure 9 expiration becomes difficult because loss of elastic recoil
reduces the volume of air that can be expired passively.
2.) There is development of large air spaces in alveoli and air spaces adjacent to
pleura because of hyperinflation.
3.) Thus the increased residual volume in the alveoli and the diminished caliber of
the bronchiole causes the part of the inspiration to be trapped in the acinus.

Multiscale Lung Modeling 16 Viraj Shah
A B
Figure 9: Air trappings in Expiration due to emphysema A) Normal expiration; B) Impaired expiration due to decreased elastic recoil (Nosek 2007)
4.) Damaged alveolar walls do not support and hold open the airways, so alveoli lose
their property of passive elastic recoil. All these contribute to collapse during
expiration.
1.5. Surface to volume (SV_Ratio) ratio change
The total alveolar surface area is a first estimate of the overall area available for gas
exchange in the lungs. The surface area is proportional to the air tissue interface.

Multiscale Lung Modeling 17 Viraj Shah
Morphometric (Haefeli-Bleuer and Weibel 1988; Verbeken, Cauberghs et al. 1992;
Kitaoka, Takaki et al. 1999) studies have consistently found an increase in the average
distance between airspace walls (mean linear intercept (Lm)) and a decrease in the
surface area of airspace wall per unit of lung volume (SV_Ratio) beginning in the third
decade of life. The decrease in SV_Ratio is approximately linear and continues
throughout life, resulting in a 25 ± 30% decrease in nonagenarians (Thurlbeck 1967;
Gillooly and Lamb 1993). Although these changes are histologically different from
emphysema (no destruction of alveolar walls), they result in similar changes in lung
compliance. These changes have been designated as "senile emphysema" (Verbeken,
Cauberghs et al. 1992). A consequence of the reduction in supporting tissues around the
airways is a tendency for the small airways (<2 mm) to collapse. Premature closure of the
airways may, therefore, occur during tidal breathing. Furthermore, the flattening of the
internal surface of the alveoli is associated with a reduction in alveolar surface (75 m2 at
age 30 yrs and 60 m2 at age 70 yrs, a reduction of 0.27 m2.yr-1) (RO 1993).
Most lungs with minimal emphysema are thought to have elements of both panacinar and
centracinar emphysema while the panacinar type predominated in the three more severely
affected lungs. The gross appreciation of emphysema generally agrees well with
decreases in SV_Ratio determined from histologic sections. The distinction between
normal lung parenchyma and mild emphysema, particularly of the panacinar type, is not
always easy to make and determination of the SV_Ratio may be the best objective
method of detecting mild alveolar destruction (Niewoehner, Kleinerman et al. 1975)
The overall elastic behavior of the lung results from the complex interrelationship of
many factors including surface tension at the air-liquid interface, the material properties

Multiscale Lung Modeling 18 Viraj Shah
of individual tissue elements, particularly collagen and elastin, and the arrangement of
these protein fibers in space and with each other. The elasticity of complex meshworks is
imparted not only by the elastic properties of individual fibers but also by their
organization. A frequently cited example to illustrate this point is the stiffness of single
nylon fibers compared to the compliant behavior of woven nylon. There are similar
examples in biological tissues (Thurlbeck 1967; Masaryk University 2005). The close
relationship observed between SV_Ratio and elasticity is consistent with the concept that
alveolar shape may be an important determinant of a lung’s elastic properties. This idea is
also supported by the observation that the relationship between SV_Ratio and elasticity
pertains for both normal and emphysematous lungs. The basic morphological processes
in aging and in emphysema are generally considered to be different. With aging the
alveolar duct increases in diameter while subtending alveoli become wider and shallower.
The outer diameter of this basic parenchymal subunit does not change significantly
(Weibel 1963). The anatomical change in emphysema is distinct in that extensive
destruction of alveolar walls occurs.

Multiscale Lung Modeling 19 Viraj Shah
Chapter 2. Previous computational models and assumptions
The modeling and functional simulation has to consider the inherent hierarchies of
biological structures, their properties and interdependencies from cellular organelles to
macroscopical anatomical structures. The lung requires an investigation of some
thousand air conducting bronchial segments, respiratory units (acini) at microscopic
resolution. A symmetric model assumes the dichotomy at each level of branching which
is not the true case (Weibel 1963) and the asymmetric model is essential for increasing
the accuracy of lung morphology (Horsfield, Dart et al. 1971). An asymmetric tree
comprises of uneven airway paths, but emulates the process of gas exchange at the
alveolar level of the lung generation.
Figure 10: A) terminal airspaces composed of hemispheres; B) Airspaces dilate concentrically shown with 2 positions; C) One hemisphere joins with another to form a spherule; D) Circumferential segment of tissue with known diameter and length (L) (Carton, Clark et al. 1964)
A model lung can be visualized as consisting of a large number of communicating spaces
of equal volume which are the alveoli. Assume that the spaces are hemispheres, (Figure

Multiscale Lung Modeling 20 Viraj Shah
10) which dilates equally and concentrically under a distending pressure, and that their
expansion is opposed only by fibers circumferentially distributed in their walls. Two
hemispheres can be grouped together as one spherule (Figure 11). The work of expansion
per hemisphere is half that of the spherule. The restraining fibers lie along segments of
great circles. To calculate work done on expansion, these can be grouped together as a
circumferential band or a segment (L) of known diameter.
2.1. Introduction
Beginning with the plastic cast of the human lung, the topology of the lung is analyzed
with classification schemes of Strahler ordering. The bronchial tree can be modeled by
regular dichotomic branching pattern, but that way it does not reproduce the cast
completely. The segments of identical order should have identical length while the distal
segments have less asymmetry. There is a relation between step size, branching angle,
length and diameter which are incorporated in the model. All these parameters provide
the database before the modeling can commence. The branching pattern of the conductive
part ends in a more dichotomic form, where the transition is from monopodial to
dichotomic branching pattern. To model the morphology of complete lung a combination
of main stem bronchi, lobes and respiratory units are modeled in unison. An iterative
method is used to determine the gas concentration. The gas concentration is described as
the mass transport due to convection, molecular diffusion and uptake. Each bronchial
segment gains mass from the distal segments and losses mass due to transport or gas
uptake in the daughter segment. The parameters used for computation are diffusion
coefficient, difference of concentration across segment and gradient, flow partitioning

Multiscale Lung Modeling 21 Viraj Shah
and dispersion coefficient. The respiratory units are classified by a SV_Ratio and gas
uptake coefficient.
For each of the segment an equation describes the conservation of mass. An iterative
method is used as a solution for the complete bronchial tree, with 10,000 time steps
necessary for a stable solution. For each segment at each time step the mass transport
equation is a differential equation which is solved by Gauss Seidel iteration. The
calculation are performed from the first order to the more distal order branches down to
the respiratory units and concentrations is stored for each segment. A stable solution is
reached after several calculations. The gas uptake can be determined at different tidal
volumes.
2.2. Previous models
User friendly software was developed, implementing previously developed models for
the structural and functional simulation of gas transport. The models previously
developed include a dichotomic branching pattern (Fleming, Halson et al. 1996; Kitaoka,
Takaki et al. 1999; Lazaridis, Broday et al. 2001; Schroeter, Fleming et al. 2002), a
representation that has its origin in the so-called trumpet model (Weibel 1963) that is
used with geometric adjustments introduced later (Erichsen, Lau et al. 1979). This is a
simplification of the lung geometry that assumes a spatially symmetric branching, a fixed
relation of the cross sections of a parent branch with the daughter branches, and a
constant length of all possible spatial trajectories between the trachea and any terminating
bronchiole. This model is self-similar regarding spatial scaling properties and as such it
reveals fractal properties. Even though the human bronchial tree, at least for the first six

Multiscale Lung Modeling 22 Viraj Shah
generations, exhibits a most symmetrical branching if compared to a wide variety of
species, irregular and asymmetric branching pattern cannot be neglected (Schlesinger and
McFadden 1981; Patra 1986). Therefore, regular symmetric models do not predict gas
flow and particle deposition reliably, and more realistic models are needed to increase
accuracy. Dichotomic branching models also tend to fill space in a spheric fashion and
cannot be adapted to the form and volume of the human lung (Sciurba, Rogers et al.). For
the local analysis of air flows, more sophisticated models are in use to show that many of
the transport processes within the airways depend quite sensitively on the geometry of the
bronchial bifurcations and the structure of the boundaries (Balásházy 1999; Darquenne
2001; Martonen 2001). But in the global modeling of the tracheobronchial tree, efforts
rely on stochastic models (Koblinger 1990; Martonen 2000) or synthesize the underlying
structure from fixed scaling laws (Kitaoka, Takaki et al. 1999) based on a small number
of anatomic examinations. These models meant to represent a mean adult lung, rather
than describing the lung of a specific individual.
2.2.1. Computer reference model
The reference model - (Schmidt, Zidowitz et al. 2004) of the bronchial tree of a normal
lung is based on high resolution computer tomography (HRCT) imaging of an in – vitro
preparation (Schmidt 2006). The software is designed on the lines of the parse tree which
has nodes which are roots, branches or leaves. The nodes are either parent or children
which get connected by branches. The lungs have a tree like structure where the trachea
is the stem and the branches arising from it are the bronchi, bronchioles, ducts and sacs. It
further handles the model parameters and the coefficients. The model takes the input

Multiscale Lung Modeling 23 Viraj Shah
parameters from the segmented data which includes the drain area, edges of the
generations, co – ordinates, length, volume, Strahler order, predicted and successive
generation for each branch. The parent branch gives rise to daughter branches and the
process continues in hierarchy.
2.2.2. Modeling gas transport
The central purpose in implementing the mass transport is to assess the significance of
the measured variation in ventilatory unit size on the uptake of inhaled gases by the gas
exchange region of the lungs. For this purpose, some simplifying assumptions were
made:
(1) The influence of radial concentration gradients on the axial dispersion of gas in
the airways is not included.
(2) The influence of radial diffusion on the axial dispersion of gas from alveolar ducts
to the adjacent alveoli is not included.
(3) The influence on transport in the airways by interactions among convection,
diffusion, and uptake in the walls is approximated by using an effective diffusion
coefficient based on empirical measurements (Scherer, Shendalman et al. 1975).
(4) Uptake at the airway walls is governed by a single mass transport coefficient that
is the same for all airways.
(5) The partitioning of flow at airway bifurcations is proportional to the distal
alveolar volume.
(6) The non alveolated conducting airways do not change dimensions with lung
inflation and deflation.

Multiscale Lung Modeling 24 Viraj Shah
Thus, the results based on the simplifying assumptions will give a conservative estimate
of the variations in ventilatory unit uptake of inhaled gases. For this implementation,
follow previous model implementation (Mercer, Anjilvel et al. 1991), which have
been developed to simulate portions of murine lungs. Hereby, the transport of gas is
governed by diffusion (mostly in the acini), convection and uptake, and for each time step
the following formulation applies.
… Equation 2
Aj Area in mm2
Cj Average gas concentration in % or µg / m3
Dj Effective diffusion coefficient in mm2 / sec
Kaw Mass transport coefficient in mm / sec
Lj Length in mm
mij Mass in segment j at time step i
SV_Ratioj Surface to volume ratio in mm-1 (mm2 / mm3)
Uj Velocity in mm / sec (> 0 for inspiration and < 0 for expiration)
ΔUC Difference in concentration across segment
ΔDC Difference in concentration gradient
Δt Difference in time between steps I and i+1 in sec
Table 1: Parameters for the jth airway segment

Multiscale Lung Modeling 25 Viraj Shah
An airway segment (j), which supplies two distal segments, is illustrated (Figure 12). The
general equation for mass balance (Equation 1) can be expressed as a function of the
fluxes across interfaces with its proximal airway segment (j - 1) and distal segments (s1
and s2). This mass balance, in terms of concentrations (C) at time step i, in Equation 2,
gives the change in mass due to transport by diffusion (D), convective flow of gas (U),
and loss from the segment by uptake at the wall (Kaw) as follows:
…Equation 3
Where,
… Equation 4
In Equation 4, A*j refers to the area of a cylinder with the same volume and length as
airway segment j and is computed in Equation 3 on the basis of the upstream cross
sectional area (Aj) and the total cross-sectional areas of the two daughter segments (As1
and As2).

Multiscale Lung Modeling 26 Viraj Shah
Figure 12: An airway bifurcation used in modeling gas concentration and uptake in airways. Airway segments s1 and s2 are daughter segments of j; C is the concentration in the segment; L is the length of the segment; A is the cross sectional area at the entrance of the segment(Mercer, Anjilvel et al. 1991)
The use of cross-sectional area in Equation 4 is mathematically equivalent to assuming
that the airway segments are tapered and allows simulations to be run for previously
published single-equivalent path models of ozone dosimetry (Miller, Menzel et al. 1978;
Miller 1985), which are specified in terms of the variations in cross-sectional area vs.
distance.
Flow partitioning to the daughter airway segments was based on the distal ventilatory
unit volume (Vvudistal) for the daughter relative to the total ventilatory unit volume
(Vvutotal) as given in the example for daughter segment s1.
… Equation 5

Multiscale Lung Modeling 27 Viraj Shah
Equation 3, when solved for Ci+l, can be used to explicitly determine the concentration in
one segment at time step i + 1 without knowledge of the concentration at the next time
step i, adjacent segments.
Gauss-Seidel iteration (Flannery B.P. 1988) is used to simultaneously solve the set of
equations giving concentration in each jth segment at the i + 1 time step. For this iteration
process (Mercer, Anjilvel et al. 1991) appropriate values for each airway segment (and
corresponding equations for the alveolar segments of each ventilatory unit) was
iteratively solved using the most recently computed values for concentration at the i+1
time step used in adjacent segments. Computations were terminated when successive
iterations through the entire system of segments changed by a maximum of 0.1 % in any
segment to simultaneously giving concentration (and corresponding equations for the
alveolar segments).
Figure 13: Conical segments used to model a ventilatory unit. Gas flow from parent airway generation is represented by leftmost segment. Alveolar ducts correspond to darkened region of each conical segment where ADj - alveolar duct cross-sectional area; A - alveolar plus alveolar duct cross-sectional area; Lj - length of each conical segment. Ventilation-induced changes in ventilatory unit size are followed by increase in length and cross-sectional area of conical segments.(Mercer, Anjilvel et al. 1991)

Multiscale Lung Modeling 28 Viraj Shah
The corresponding mass transport equation for each segment of the ventilatory unit is
similar to that given for the airways. As illustrated (Figure 17), the model for the
ventilatory unit is specified by a series of tapered cones where the taper is specified by
the measured changes in cross-sectional area vs. distance for the alveolar ducts and
alveolar subdivisions.
The principal differences from the airway segment equation correspond to the addition of
an alveolated compartment around the alveolar ducts and the variation in length and
cross-sectional area of each segment with ventilation. In this model, gas convection and
diffusion are assumed to occur at the interfaces between segments formed by the alveolar
ducts (cross-sectional area, AD) while the total volume of the segment includes both-the
alveolar and alveolar duct subdivisions (volume-equivalent cross-sectional area, A*).
Chemical reactions occur at the alveolar walls, which are distributed in the segment
volume. The amount of uptake is proportional to the total alveolar surface per segment,
which depends on the volume of the segment (A*L), and the alveolar surface-to-volume
ratio (SV_Ratio) times a mass transfer coefficient (Kalv)
Initially, the concentration in all airway and alveolar segments is assumed equal to the
tissue concentration of the gas (which is CV for oxygen). The concentration at the airway
segment that was the input to the model was fixed at 21% for O2. To obtain a solution for
uptake by the ventilatory units, the simulation is run until the changes in uptake by the
ventilatory units in successive breaths was 1%. A concentration of 5.26% was used for
tissue oxygen back pressure (CV). The uptake of oxygen in the airways was assumed to
be negligible. A molecular diffusion coefficient of 17.3 mm2 /sec was used for O2.

Multiscale Lung Modeling 29 Viraj Shah
2.2.3. Qt GUI features
In order to develop a user friendly interface for the computational modules of this
software, we have considered QT. It has the features for robustness and easy object
oriented programming toolkits which add to the reusability. QT supports the development
of cross platform GUI applications with its “write once, compile anywhere” approach.
Using a single source tree and a simple recompilation, applications can be written for
many operating systems. Qt introduces a unique inter-object communication mechanism
called “signals and slots”. Qt applications can be built visually using Qt Designer, a
flexible user interface builder with support for IDE (Integrated Development
Environment) integration (Trolltech 2007).
2.2.3.1. QObject Class
The QObject class is the base class of all Qt objects and is the heart of the Qt object
model. The central feature in this model is a very powerful mechanism for seamless
object communication called signals and slots. QObjects organize themselves in object
trees. When a QObject is created with another object as parent, the object will
automatically do an insertChild ( ) on the parent and thus show up in the parent's children
( ) list. The parent takes ownership of the object i.e. it will automatically delete its
children in its destructor. Every object has an object name ( ) and can report its
className ( ) and whether it inherits ( ) another class in the QObject inheritance
hierarchy. The QObject macro is mandatory for any object that implements signals, slots
or properties and also the moc program (Meta Object Compiler) has to be run on the
source file. Using this macro in all subclasses of QObject regardless of whether or not

Multiscale Lung Modeling 30 Viraj Shah
they actually use signals, slots and properties, prevents functions to exhibit undefined
behavior.
2.2.3.2. Signals and Slots:
Signals and slots are used for communicating between objects. The signal/slot
mechanism is a central feature of Qt and probably the part that differs most from other
toolkits. In GUI programming, there are changes in one widget which are to be notified to
other widgets. The objects of any kind should be able to communicate with one another.
Older toolkits achieve this kind of communication using callbacks. A callback is a pointer
to a processing function. Callbacks have two fundamental flaws. Firstly they are not type
safe, where there is no certainty that the processing function will call the callback with
the correct arguments. Secondly the callback is strongly coupled to the processing
function since the processing function must know which callback to call. An alternative
to the callback technique is signals and slots. A signal is emitted when a particular event
occurs. Qt has widgets which have many pre-defined signals and there can be addition of
new subclasses. A slot is a function which is called in response to a particular signal. It
even has widgets which have many pre-defined slots and there are benefits of adding
slots so that handling any signal can be easily maneuvered. The signals and slots
mechanism is type safe, where the signature of a signal must match the signature of the
receiving slot. In fact a slot may have a shorter signature than the signal it receives
because it can ignore extra arguments. Since the signatures are compatible, the compiler
helps to detect type mismatches. Signals and slots are loosely coupled where a class
which emits a signal is independent of the slots which receive the signal. Qt's signals and

Multiscale Lung Modeling 31 Viraj Shah
slots mechanism ensures that if a signal is connected to a slot, the slot will be called with
the signal's parameters at the right time. Signals and slots can take any number of
arguments of any type. All classes that inherit from QObject or one of its subclasses (e.g.
QWidget) can contain signals and slots. Signals are emitted by objects when they change
their state in a way that may be interesting to the outside world. This is all the object does
to communicate. It is independent about whether anything is receiving the signals it
emits. This is true information encapsulation, and ensures that the object can be used as a
software component. Slots can be used for receiving signals, but they are also normal
member functions. Just as an object does not know if anything receives its signals, a slot
does not know if it has any signals connected to it. This ensures that truly independent
components can be created with Qt. There can be as many signals connected to a single
slot, and a signal can be connected to as many slots after that. It is even possible to
connect a signal directly to another signal which will emit the second signal immediately
whenever the first is emitted. Together, signals and slots make up a powerful component
programming mechanism.
2.2.4. Benefits of QT for lung modeling
The library provided by Qt has the modules for functionality, GUI classes and Open GL.
Qt designer has an advantage to use icons to stand for data which have a powerful GUI
layout and forms builder. It has advanced features for preview, widget layout and
property editor. For designing the user interface for changing the simulation parameters,
statistics layout, results, parameters and the graphics parameters Qt has the versatility to
design each window with a new feature within it.

Multiscale Lung Modeling 32 Viraj Shah
It provides for easier memory management because child objects are automatically
deleted when their parents are, this approach largely combines the ease of use of garbage
collected languages with the efficiency and lower resource consumption of C++. GUI
layout can specify which parts of the layout should be grouped, which should stretch and
which should be constrained.
2.3. Design of user interface and steps in simulation
Figure 14: Flowchart of the simulation
1. Load the tree geometry: Open the data file as shown (Figure 15) using the File
option menu. After hitting that option the browser takes to the root directory of

Multiscale Lung Modeling 33 Viraj Shah
software, where the data files are stored. Using the select tool, select one of the
files needed for the operation. The data files are in the format of the segmented
tree data structures with the number of nodes and edges. Within the structure of
the file the nodes and edges are separated for the number of generations and
ordering of the tree structure. QT uses this organization to extract the data from
the input files.

Multiscale Lung Modeling 34 Viraj Shah
Figure 15: Top) Graphical User Interface (GUI) for loading a tree geometry using the software Bottom) Graphical User Interface (GUI) for opening a data file using the software
Depending on the complexity of the data the number of generations in the display shall
display the hierarchy of the tree structure.
2. Highlight the path for modeling the gas uptake: Select the path as shown in figure
16 for simulation and use the palette of controls to perform additive, subtractive,
logical operations, branch selection, pan, zoom, x, y and z rotation at each level of
branching.

Multiscale Lung Modeling 35 Viraj Shah
Figure 16: A tree structure of the lung loaded using the software and the path of gas flow on the tree structure highlighted using the color blue
3. Select the simulation parameters: Using the GUI, select the parameters for the
simulation as shown in figure17. Except the surface to volume ratio all the
physiological parameters remain constant for the test conditions of emphysema.
Change the acinus geometry in some instances to check for the dimensional
changes.

Multiscale Lung Modeling 36 Viraj Shah
A: Physiological Parameters B: Acinus Geometry
Figure 17: A) Describes the lung physiology parameters; B) Describes the respiratory unit (acinus geometry)
4. Start the simulation: Start the simulation for the gas uptake and concentration
profiles at inspiration and expiration. As seen from figure 18 the display window
shows the time span of the simulation with an estimated wait time for execution,
after which it displays a successful completion of the process.

Multiscale Lung Modeling 37 Viraj Shah
Figure 18: Display of approximate duration of simulation after the process is initiated
5. Open the result file: After the simulation is completed go to the file menu and
open the output file as shown in figure 19 with concentration profiles for the
entire tree path of the simulation. It holds information for the number of
generations of acini and number of branches.

Multiscale Lung Modeling 38 Viraj Shah
Figure 19: Left) Open the result file; Right) Select the output file to open
6. Analyze and write the results: Select the analyze option and write the results for 0
to 4 sec interval as shown in figure 20 or whatever duration of breathing cycle the
process was initialized for the branches and all the acini for selected branches.

Multiscale Lung Modeling 39 Viraj Shah
Figure 20: Left) Write the results; Right) Results data analysis time duration and acini data selection option
2.4. Example of modeling a normal lung
The log file gives the information for the parameters selected for the simulation. It gives
the file selected for performing the operation, end time of each respiratory cycle in
seconds (End time), time step for each simulation (Delta T), actual output delta in
seconds (OutputDelta), initial concentration of the trachea in percentage of gas
(C_Trachea), tidal volume of intake in milliliters (mL) (V_tidal), maximum speed in
trachea in mm/sec (U_trachea), period of one breathing cycle which is inspiration with
expiration in seconds (Period), alveolar diffusion coefficient in mm2/ sec (D_alv),
surface to volume ratio in mm-1 (SV_Ratio), venous backpressure in mmHg (C_v), initial
concentration of gas in % (C_initial) and the total time of simulation in seconds.

Multiscale Lung Modeling 40 Viraj Shah
Figure 21: Log file of the simulation with the model parameters and duration of simulation
2.7 Emphysema
In order to extend the model of a normal lung with a simulation of an emphysematic
lung, the structural properties of the respiratory units needs to be changed. The required
data was published in - (Coxson, Rogers et al. 1999) and was collected from a group of
patients with CT scan analysis performed and SV_Ratio was collected from patients with

Multiscale Lung Modeling 41 Viraj Shah
control and emphysema groups. The separation into three groups was based on the
percent of the lung that was determined to be emphysematous using the “density mask”
technique. For the process of simulations the below data (Table 2) is used for the surface
to volume ratio for the normal, mild emphysema and severe emphysema groups. This
entire setup is used to check the change in concentration at each stage of disease with the
influence from the change in surface to volume ratio which is the main change seen in
emphysema and aging which has the similar modification. The data used for the
measured cases is used for the calculation.
Table 2: Quantitative analysis of the lung (Coxson, Rogers et al. 1999)
2.5. Measurement of histological and micro CT on lung emphysema
In addition to the data provided in the literature, we also measured SV_Ratio in
histological and micro CT images. The image data has been used previously to perform a

Multiscale Lung Modeling 42 Viraj Shah
visualization of the fine structure of the bronchial tree (Watz, Breithecker et al. 2005).
Micro tomography uses X - rays to visualize smaller objects with cross section of the
pixels in the micrometer range to give high spatial resolution and exceptional speed of
operation. The internal structures are reconstructed as a set of flat cross sections where
the process is non destructive. The micro CT of normal lung in fig. 27.1 clearly
demarcates the alveoli of a normal lung parenchyma, where a respiratory bronchiole is
dividing two alveolar ducts.
1 2
3 4
Figure 22: 1) Micro CT of normal lung; 2) Histogram of figure 1; 3) Contrast stretched figure 2; 4) Thresholded figure 3

Multiscale Lung Modeling 43 Viraj Shah
The successive figures show the histogram of the original, followed by the contrast
stretching and thresholding with Image J toolkit which gives a SV_Ratio of 27.59 mm-1
for a normal lung. The alveoli are enlarged and the alveolar septa are missing in fig. 28
which highlights the features of emphysema. The SV_Ratio is calculated by thresholding
the original image using Image J toolkit and then calculating the perimeter and area of the
fig 28.3 which gives a ration of 4.84 mm-1.
Figure 23: 1) Micro CT of Centrilobular emphysema; 2) Thresholded figure 1; 3) Results of the SV_Ratio for figure 2
31 2
The data measured is along the lines of the measured data (Coxson, Rogers et al. 1999)
and the SV_Ratio from the published data is used for the simulation in the model.
2.6. Extension of user interface to handle emphysema
Change in the surface to volume ratio is the main characteristic of emphysema (see figure
8). The SV_Ratio we are using for our simulations changed from 34.6 mm-1 to 16.5 mm-1
to 4.3 mm-1 for normal to mild emphysema to severe emphysema conditions of the lungs
respectively. We assumed a situation of panacinar emphysema (see figure 8c) with equal

Multiscale Lung Modeling 44 Viraj Shah
changes in all segments of the respiratory model. Thus the change in SV_Ratio is entered
as a single parameter (see figure 24). The simulation process is repeated for 20 breathing
cycle for achieving a stable condition.
Figure 24: Change in SV_Ratio to handle emphysema with other parameters on the GUI interface

Multiscale Lung Modeling 45 Viraj Shah
Chapter 3. Results
3.1. Results of gas transport simulation in normal Lung
In the Figure 25, the normal lung gas uptake for a single breathing cycle with a tidal
volume of 500 mL and surface to volume ratio of 34.6mm-1 is compared to an
emphysematic lung with a surface to volume ratio of 16.5 mm-1 and tidal volume of
250mL.
Figure 25: A) Concentration (%) to time (sec) for a healthy lung with a mean +/- std. dev of 3.753+/- 6.10; B) Concentration (%) to time (sec) for an Emphysematic lung with a mean +/- std. dev of 2.87 +/-5.99; C) Difference in Concentration (%) to time (sec) from a healthy to an emphysematic lung with a mean +/- std. dev of 0.883+/-1.40

Multiscale Lung Modeling 46 Viraj Shah
3.2. Results for emphysematic lungs
Figure 26: A) Surface to volume ratio to disease conditions of emphysema (Coxson, Rogers et al. 1999)
In fig. 26 the measured and predicted data (Coxson, Rogers et al. 1999) for normal lung
and emphysematic lung with change is surface to volume ratio are compared. In fig. 30
and 31 the repeatability of software is tested for 20 repetitions and the variability for 7
generations of acini is tested for mild and severe conditions of emphysema. The mild

Multiscale Lung Modeling 47 Viraj Shah
emphysema and severe emphysema have a SV_Ratio of 16.5 mm-1 and 4.5 mm-1
respectively.
1 2
3 4
Figure 27: 1, 3) Difference of each repetition with the first repetition showing the repeatability of software output; 2, 4) Change in concentration for 7 generation of acini with a mean +/- std. of 0.81+/- 0.44

Multiscale Lung Modeling 48 Viraj Shah
1 2
43
Figure 28: 1, 3) Difference of each repetition with the first repetition showing the repeatability of software output; 2, 4) Change in concentration for 7 generation of acini with a mean +/- std. of 0.87 +/- 0.46

Multiscale Lung Modeling 49 Viraj Shah
Figure 29: Difference in normal lung (T_V is 500 mL and S_V ratio is 34.6 mm-1) to emphysematic lung (T_V is 250 mL and S_V ratio is16.5 mm-1) concentration with 1.4 +/- 0.95 (mean +/- std. dev.)
In figures 27 and 28 the reproducibility of the software is tested for 20 repetitions. The
variability is none in the software. The mild emphysema has SV_Ratio of 16.6 mm-1 and
severe emphysema has 4.3 mm-1 where there is change in concentration for an acini
tested. The branches 23, 29 and 88 belong to the 5th, 8th and 12th generations respectively
for fig. 32 and 33. The branches from the 5th and 8th generations belong to the upper and
lower lobes of the right lung respectively and branches from the 12th generation belong to
the left lower lobe.
Minute Volume = [Tidal volume × Respiration rate]… Equation 6
Where, respiration rate is 15 breaths / min

Multiscale Lung Modeling 50 Viraj Shah
Figure 30: Difference in normal lung (T_V is 500 mL and S_V ratio is 34.6 mm-1) to emphysematic lung (T_V is 500 mL and S_V ratio is16.5 mm-1) concentration 0.757 +/-0.79 (mean +/- std. dev.)
The alveolar ventilation rate which is the volume of entering respiratory bronchiole and
alveoli during a minute changes from 5.25 mL/min to 1.5 mL/min in emphysema. So
when the dead space to tidal volume ratio reduces the effective ventilation reduces.
Alveolar Ventilation Rate (mL/min) = [(Tidal volume – Dead space) × (Respiratory rate
/ min)] … Equation 7
In fig 31 to 33 there are 7 generations of acini compared for concentration to time from a
normal to mild emphysema to severe emphysema conditions. The fig. 44 and 45 compare
the difference of normal lung to emphysematic conditions and fig 46 shows the ratio of
disease conditions to normal lung for first and twentieth breathing cycles.

Multiscale Lung Modeling 51 Viraj Shah
3.2.1. Emphysematic data with change in SV_Ratio
Figure 31: Normal lung uptake with a men +/- std. dev. of 2.57 +/- 0.11 for acini segment 98 and 7 generations of acini for 20 breathing cycles (4sec *20 = 80 sec)

Multiscale Lung Modeling 52 Viraj Shah
Figure 32: Uptake for mild emphysema disease condition with a mean +/- std. dev. of 1.55 +/- 0.05 for acini segment 98 and 7 generations of acini for 20 breathing cycles (4sec *20 = 80 sec)

Multiscale Lung Modeling 53 Viraj Shah
Figure 33: Severe emphysema uptake with a mean +/- std. dev. of 0.48 +/- 0.01 for acini segment 98 and 7 generations of acini for 20 breathing cycles (4sec *20 = 80 sec)

Multiscale Lung Modeling 54 Viraj Shah
Figure 34: Left to right, normal lung (12.08 +/- 0.28), mild emphysema (13.81 +/- 0.24) and severe emphysema (15.39 +/-0.21), acini segment 98 and 7 generations of acini for the 20th breathing cycle

Multiscale Lung Modeling 55 Viraj Shah
Figure 35: Difference in the normal lung to mild emphysema of 1.74 +/- 0.06 for 20th breathing cycle of 4 sec (The negative sign is due to mild emphysema being greater in concentration compared to normal lung)

Multiscale Lung Modeling 56 Viraj Shah
Figure 36: Difference in normal lung to severe emphysema of 3.31 +/- 0.12 for 20th breathing cycle of 4 sec (The negative sign is due to severe emphysema being greater in concentration compared to normal lung)

Multiscale Lung Modeling 57 Viraj Shah
1 2
3 4
Figure 37: Concentration ratios for 1) Normal to mild 1st breathing cycle with a mean +/- std of 1.2 +/- 0.3; 2) Normal to mild for 20th breathing cycle with a mean +/- std of 0.87 +/- 0.005; 3) Normal to mild for 1st breathing cycle with a mean +/- std of 1.833 +/- 1.88; 4) Normal to mild for 20th breathing cycle with a mean +/- std of 0.78 +/- 0.009

Multiscale Lung Modeling 58 Viraj Shah
In figure 34 the normal lung with SV_Ratio of 34.3 mm-1, mild emphysema with a
SV_Ratio of 16.5 mm-1 and severe emphysema with an SV_Ratio of 4.3 mm-1 is
calculated for the 20th breathing cycle for an acini. For figure 35 and 36 the difference in
normal to mild and severe cases of emphysema are compared for a single acini. For
figure 35 there is ratio of normal to mild and normal to severe compared for the 1st and
20th breathing cycle.

Multiscale Lung Modeling 59 Viraj Shah
Chapter 4. Discussion The important outcomes from this study are the changes in surface to volume ratio and its
effects on the uptake and concentration profiles in structural segments of the bronchial
tree. The movement of gases across the respiratory surface occurs mainly by diffusion
and some part by convection and direct uptake. The rate of diffusion is proportional to the
surface area across which the diffusion occurs. Since the alveoli do not collapse
completely, some air remains in the lung and is called as the residual volume. Thus the
lungs are unable to empty with each breath cycle and there is inhaled air mixed with the
residual air. So, the oxygen concentration in alveoli is considerably less than the
atmosphere. The reduction in surface area decreases the area for gas exchange for
conditions of emphysema. It greatly affects dead space, alveolar gas uptake and effective
ventilation.
In emphysema the tidal volume reduces and the surface to volume ratio decreases
considerably due to the reduced alveolar functional surface area. The air coming in the
trachea is increased with the change of tidal volume from 500mL to 250 mL, but the
residual volume increase due to air trapping in the alveoli.
All lungs judged to contain emphysema had an SV_Ratio of less than 16.5 mm-1 while
the SV_Ratio in all nonemphysematous lungs was greater than 25.6 mm-1.
In the Figure 29, the normal lung gas concentration is 3.75 +/- 6.10 % with the tidal
volume as 500 mL and surface to volume ratio is 34.6mm-1, while in the emphysematic
lung the surface to volume ratio is 16.5 mm-1 and tidal volume is 250mL and the gas
concentration is 2.87 +/- 5.99 % and the difference in normal to emphysematic
concentration is of 0.88 +/- 1.4 %. Thus due to emphysema the available gas for

Multiscale Lung Modeling 60 Viraj Shah
exchange reduces by 0.88% for the first breathing cycle and it increase for the successive
breathing cycles.
As seen from figure 32 and 33 the functional residual capacity which is related to
expiration increases with the progress of emphysema. The residual air in the lungs also
increases with the progress of emphysema. Since the tidal volume changes from 500 mL
to 250 mL the minute ventilation changes from 6 liters (L) to 3.75 L for normal to
emphysematic conditions.
The software output is very stable with no variability seen for 20 repetitions. The
concentration in the 7 generations of acini changes from 0.81% to 0.87% for mild to
severe conditions of emphysema. Thus the gas exhaled is affected with the change in
surface to volume ratio parameters.
Going from the normal lung to emphysematic conditions the uptake of the gas in the acini
decrease from, 2.57 % to 1.55 % to 0.48 % for normal, mild emphysema and severe
emphysema conditions respectively over 20 breathing cycles. For the twentieth breathing
cycle the concentration of gas in the lungs increases from 12.08 % for normal to 13.81 %
for mild emphysema to 15.39 % for sever emphysema conditions. This way the residual
volume of the lung increases and uptake decreased causing shortness of breath even with
mild exercises due to less oxygen available for circulation. From the first breathing cycle
to twentieth breathing cycle the ratio of normal to mild changes from 1.19 to 0.87 and for
normal to severe conditions of emphysema changes from 1.83 to 0.79. Thus the
efficiency reduces for conditions of emphysema from 13 % to 21 % for mild and severe
conditions of emphysema respectively.

Multiscale Lung Modeling 61 Viraj Shah
It takes 38 hours of computational time (134454 sec) for running a simulation of 20
breathing cycles of 4 sec each. With the complexity of hierarchy and increase in the
number of breathing cycles the time required for simulation increases.
4.1. Future extensions of the model:
Improvements in the model with more realistic parameters of elasticity and pressure shall
make the model handle more conditions of diseases. There is a significant decrease in
SV_Ratio with age even in nonemphysematous lungs (Thurlbeck 1967). Although
functional change associated with the decrease in SV_Ratio is very similar in both the
aged and the emphysematous lung. The elastic behavior of the aging lung also changing
due in part or solely to other factors including changes in the mechanical properties of
individual fibers. Thus with the incorporation of elasticity parameters the conditions of
aging can be simulated more realistically using this model. It shall be incorporated as a
GUI feature like the SV_Ratio which can allow to evaluate aging and emphysema which
have the similar pattern of effects. Pulmonary resistance is overall affetced in emphysema
and it affects more of expiration than of inspiration.

Multiscale Lung Modeling 62 Viraj Shah
List of References 1. Anderson, A. E., Jr. and A. G. Foraker (1962). "Relative dimensions of
bronchioles and parenchymal spaces in lungs from normal subjects and emphysematous patients." Am J Med 32: 218-26.
2. ATS, A. T. S. (1962). "Chronic bronchitis, asthma and pulmonary emphysema: a statement by the Committee on Diagnostic Standards for Nontuberculous Respiratory Diseases." American review of respiratory disease 85: 762 - 768.
3. Balásházy, e. a. (1999). "Computation of local enhancement factors for the quantification of particle deposition patterns in airway bifurcations " Journal of Aerosol Science 30(2): 185-203.
4. Boren, H. (1962). "Alveolar fenestrae: relationship to the pathology and pathogenesis of pulmonary emphysema." American review of respiratory disease 85: 328-344.
5. Carton, R. W., J. W. Clark, et al. (1964). "Estimation of Tissue Elasticity of the Lung." J Appl Physiol 19: 236-42.
6. Coxson, H. O., R. M. Rogers, et al. (1999). "A quantification of the lung surface area in emphysema using computed tomography." Am J Respir Crit Care Med 159(3): 851-6.
7. Darquenne, C. (2001). "A realistic two-dimensional model of aerosol transport and deposition in the alveolar zone of the human lung " Journal of Aerosol Science 32(10): 1161 - 1174.
8. Dunnil (1962). "Postnatal growth of the lung." Thorax 17(329 - 333): 17.
9. Erichsen, J., D. Lau, et al. (1979). "[Effect of methylprednisolone on bacterial infection of the peritoneal cavity in the guinea pig]." Chirurg 50(6): 370-4.
10. Flannery B.P., T. S. A., Vetterling W. T. (1988). Numerical Recipes in C, Cambridge University Press.

Multiscale Lung Modeling 63 Viraj Shah
11. Fleming, J. S., P. Halson, et al. (1996). "Three-dimensional description of pulmonary deposition of inhaled aerosol using data from multimodality imaging." J Nucl Med 37(5): 873-7.
12. Gillooly, M. and D. Lamb (1993). "Airspace size in lungs of lifelong non-smokers: effect of age and sex." Thorax 48(1): 39-43.
13. Gillooly, M. and D. Lamb (1993). "Microscopic emphysema in relation to age and smoking habit." Thorax 48(5): 491-5.
14. Greer, P. H. (2007). "Respiration." from http://www.cayuga-cc.edu/people/facultypages/greer/biol204/resp3/resp3.html.
15. Haefeli-Bleuer, B. and E. R. Weibel (1988). "Morphometry of the human pulmonary acinus." Anat Rec 220(4): 401-14.
16. Horsfield, K. and G. Cumming (1968). "Morphology of the bronchial tree in man." J Appl Physiol 24(3): 373-83.
17. Horsfield, K., G. Dart, et al. (1971). "Models of the human bronchial tree." J Appl Physiol 31(2): 207-17.
18. Kitaoka, H., R. Takaki, et al. (1999). "A three-dimensional model of the human airway tree." J Appl Physiol 87(6): 2207-17.
19. Koblinger, L. H., W (1990). "Monte Carlo modeling of aerosol deposition in human lungs. Part I: Simulation of particle transport in a stochastic lungs structure." Journal of Aerosol Science 21(5): 661 - 674.
20. Kriete, A. (1998). "Form and function of mammalian lung: analysis by scientific computing." Adv Anat Embryol Cell Biol 145: III-IX, 1-105.
21. Lazaridis, M., D. M. Broday, et al. (2001). "Integrated exposure and dose modeling and analysis system. 3. Deposition of inhaled particles in the human respiratory tract." Environ Sci Technol 35(18): 3727-34.

Multiscale Lung Modeling 64 Viraj Shah
22. Machen, P. F. P. T. (2007). "Molecular and Cell Biology 136 ", from http://mcb.berkeley.edu/courses/mcb136/topic/Respiration/SlideSet3/Resp3.htm.
23. Martonen, T., et. al. (2000). "Lung models:strengths and limitations." Respir Care 45(6): 712 - 736.
24. Martonen, T. B. (2001). Medical Applications of Computer Modelling: The Respiratory System, WIT Press.
25. Masaryk University. (2005). from http://www.med.muni.cz/patfyz/pdf/new/lung_new.pdf.
26. Mercer, R. R., S. Anjilvel, et al. (1991). "Inhomogeneity of ventilatory unit volume and its effects on reactive gas uptake." J Appl Physiol 70(5): 2193-205.
27. Mercer, R. R. and J. D. Crapo (1987). "Three-dimensional reconstruction of the rat acinus." J Appl Physiol 63(2): 785-94.
28. Miller, F. J., D. B. Menzel, et al. (1978). "Similarity between man and laboratory animals in regional pulmonary deposition of ozone." Environ Res 17(1): 84-101.
29. Miller, F. J. e. a. (1985). "A model of the regional uptake of gaseous pollutants in the lung. I. The sensitivity of the uptake of ozone in the human lung to lower respiratory tract secretions and exercise." Toxicol Appl Pharmacol 79(1): 11-27.
30. Nagai A, Y. I., Takizawa T, Thurlbeck WM (1991). "Alveolar attachments in emphysema of human lungs." American review of respiratory disease 144: 888-891.
31. Niewoehner, D. E., J. Kleinerman, et al. (1975). "Elastic behavior of postmortem human lungs: effects of aging and mild emphysema." J Appl Physiol 39(6): 943-9.
32. Nosek , T. M. (2007). Essentials of human physiology. Georgia.

Multiscale Lung Modeling 65 Viraj Shah
33. Patra, A. L. (1986). "Comparative anatomy of mammalian respiratory tract: the nasopharyngeal region and the tracheobronchial tree." Journal of Toxicology and Environmental Health(17): 163-174.
34. Petty, T. L., G. W. Silvers, et al. (1986). "Radial traction and small airways disease in excised human lungs." Am Rev Respir Dis 133(1): 132-5.
35. Pratt, P. C., A. Haque, et al. (1961). "Correlation of postmortem function and structure in normal and emphysematous lungs." Am Rev Respir Dis 83: 856-65.
36. RO, C. (1993). Pulmonary Disease in the elderly patient. New York, Marcel Dekker.
37. Roy, D. R. (2007). "Pathophysiology of emphysema." from http://www.geocities.com/drroy5/Emphysema1.JPG.
38. Saetta, M., H. Ghezzo, et al. (1985). "Loss of alveolar attachments in smokers. A morphometric correlate of lung function impairment." Am Rev Respir Dis 132(4): 894-900.
39. Scherer, P. W., L. H. Shendalman, et al. (1975). "Measurement of axial diffusivities in a model of the bronchial airways." J Appl Physiol 38(4): 719-23.
40. Schlesinger, R. B. and L. A. McFadden (1981). "Comparative morphometry of the upper bronchial tree in six mammalian species." Anat Rec 199(1): 99-108.
41. Schmidt, A. (2006). "Unpublished."
42. Schmidt, A., S. Zidowitz, et al. (2004). "A digital reference model of the human bronchial tree." Comput Med Imaging Graph 28(4): 203-11.
43. Schroeter, J. D., J. S. Fleming, et al. (2002). "A computer model of lung morphology to analyze SPECT images." Comput Med Imaging Graph 26(4): 237-46.
44. Schulze, F. E. (1871). "Die Lungen, in: Stricker, S. (Ed.)."

Multiscale Lung Modeling 66 Viraj Shah
45. Sciurba, F. C., R. M. Rogers, et al. (1996). "Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema." N Engl J Med 334(17): 1095-9.
46. Snider, G. L. e. a. (1985). "The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop." Am Rev Respir Dis 132(1): 182-5.
47. Tawhai, M. H. (2001). An anatomically based mathematical model of the human lungs, applied to gas mixing and water vapour and heat transport. Department of Engineering Science, School of Engineering. New Zealand, The University of Auckland. Doctor of Philosophy.
48. Thurlbeck, W. M. (1967). "The internal surface area of nonemphysematous lungs." Am Rev Respir Dis 95(5): 765-73.
49. Thurlbeck, W. M. and N. L. Muller (1994). "Emphysema: definition, imaging, and quantification." AJR Am J Roentgenol 163(5): 1017-25.
50. Trolltech. (2007). "Whitepapers." from http://trolltech.com/products/qt/learnmore/whitepapers.
51. Underwood , E. E. (1970). Quantitative Stereology, Addison-Wesley Pub. Co.
52. University, M. (2005). from http://www.med.muni.cz/patfyz/pdf/new/lung_new.pdf.
53. Verbeken, E. K., M. Cauberghs, et al. (1992). "The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects." Chest 101(3): 793-9.
54. Watz, H., A. Breithecker, et al. (2005). "Micro-CT of the human lung: imaging of alveoli and virtual endoscopy of an alveolar duct in a normal lung and in a lung with centrilobular emphysema--initial observations." Radiology 236(3): 1053-8.
55. Weibel, E. R. (1963). Morphometry of the Human Lung. Berlin, Springer-Verlag.

Multiscale Lung Modeling 67 Viraj Shah
56. WHO, W. H. O. (1961). "Chronic cor pulmonale: report of an expert committee." Health Organization technical report series 213.
57. Wilmot C Ball Jr., M. D. (1996). "Interactive respiratory Physiology." Johns Hopkins School of Medicine.
58. Wright , J. L. H., J.E;Wiggs, B;Pare, P.D;Hogg, J.C (1989). "Airway inflammation and peribronchiolar attachments in the lungs of non smokers, current and ex-smokers." Lung 167(1): 1432-1750.

Multiscale Lung Modeling 68 Viraj Shah
Vita VIRAJ N. SHAH
3175, JFK Blvd, Apt1306, Philadelphia, PA-19104 • Phone: 267-357-3494 • E-mail:[email protected]
ABOUT MYSELF Team player with expertise in instrumentation, software and algorithm development, statistical and quantitative analysis, six sigma statistics and mathematical modeling OBJECTIVE Zeal to apply my analytical, mathematical and programming skills for developing new systems
SKILLS Software Development: Designed, developed and coordinated software scripts and algorithms Programming Languages: C/C++, VB/VC++ 6.0, MATLAB/MathCAD, Qt Operating Systems: Windows Vista, XP, ME, 2000, 98, 95 Web Technology: HTML, Wikipedia/TWiki Applied Mathematics: Mathematical modeling using Monte Carlo simulation, Black Scholes algorithm, differential equations, regression analysis, ANOVA, probability theory, neural models
Research and Development: Thesis on Lung Model development on VC.net platform to simulate and predict unforeseen conditions; Affine transforms, statistical reteach and Monte Carlo simulation for an Accuracy model EDUCATIONAL QUALIFICATIONS • Masters of Science in Biomedical Engineering, Drexel University, PA Sep’05 – Dec’07
Thesis: Investigate changes in the size of respiratory units (acini) under emphysema in a model of the human lung by computer simulation (GPA 3.63/4.00)
• Bachelor of Science in Biomedical Engineering, Mumbai University, INDIA Sep’01 - May’05 (GPA 3.75/4.00) WORK EXPERIENCE • Co-op at Kulicke & Soffa Industries, Inc, Fort Washington, PA, United States Sep’06 – Sep’07 • Achieved a sub-system accuracy of less than 1.75 micrometer, 3-sigma standard deviation for a
35 micrometer IC chip interconnection by eliminating error sources • Performed reversal technique for the Table Map using Monte Carlo simulations using MATLAB
to achieve self calibration for a wire bonder • Developed Accuracy model using MATLAB and Excel software’s to eliminate error sources at
the scale of a micron • Developed an enterprise Wikipedia for collaboration, knowledge management, project planning
for various locations around the world • Prepared a statistical reteach algorithm by deriving mathematical equations with 3 sigma analyses
in MATLAB to correct the placement error in a wire bonding equipment
• Internship at Philips Medical Systems India Pvt. Ltd, Mumbai, India Jun’04 - Nov’04 • Worked as a Field Service Engineer and installed CathLabs, MRI’s, CT scans, Ultrasound
machines, cardiac monitors and defibrillators. • Performed planned maintenance and troubleshooting for breakdowns and malfunctions

Multiscale Lung Modeling 69 Viraj Shah
• Accomplished liquid nitrogen refill for 3 Tesla Magnetic Resonance Imaging equipment
• Drexel University, Philadelphia, PA • Research Assistant, Neuroengineering Department Nov’05 - Sep’06 • Designed and developed neural mass models using Runga Kutta techniques on MATLAB for
simulating schizophrenia and brain interconnection techniques
• Teaching Assistant, Bioscience and Biotechnology Department Jan’06 - Jun’06 • Taught various protein quantification assays and protein extraction techniques to a class size
of 20 students • Conducted Lab sessions with safety techniques, tutored students to obtain and analyze data
using Microsoft-Excel package
PROJECT EXPERIENCE • Consultant to prepare Annual Corporate Social Responsibility(CSR) report Jun’07 – Present
Compiled a CSR report which encompasses community development, diversity, marketplace practices, accountability and environmental stewardship for Kulicke & Soffa Industries, Inc
• FDA report to perform risk management and design analysis on medical devices Mar’07 – Jun’07
Prepared a report meeting the FDA 510k, design and safety guidelines for Non-Invasive Insulin Delivery using nano-particles for the treatment of diabetes
• Black Scholes method to analyze futures stock prices Mar’07 – May’07 • Prepared a Visual Basic script in Excel using Black Scholes formula to forecast the prices of
stocks using a Monte Carlo simulation techniques • Used binary tree approach for accounting the option trades
• DNA computers Oct’05 - Dec’06 • Prepared a literature review on traveling salesman problem using DNA computers and
submitted a research paper with the applications and future of DNA computers • Analyzed the functioning of DNA computer with a simple mathematical example
• Electroencephalograph (EEG)recording and infrared transmission Dec’04 - May’05 Designed a system for transmitting EEG signal over infrared and to check the EEG pattern and analyze them using MATLAB
• Institutional Review Board (IRB) Jan’06 - Mar’06 Drafted an IRB for a system with Electrocculography (EOG) for animal and human research protocols
PROFESSIONAL SKILLS Part of Drexel Delegation to United Nations HQ, New York City Aug’07
• Analyzed the Millennium Development Goals (MDG’s) to be achieved by 2015 • Participated in workshops and discussions with speakers from all around the world
Vice President Drexel IEEE Graduate Forum May’06 – May’07
• Conducted workshops, ad hoc events, career fairs, invited prominent speakers and arranged Professional Socials for the Drexel University Graduate Students

Multiscale Lung Modeling 70 Viraj Shah
EXTRA CURRICULAR • Performed for Drexel Hungama and Drexel Dhinchak dance troops which perform annual
shows for Drexel University • Completed Mountaineering, Camping, Trekking and river rafting activities with Lion Hearts
Adventure Group, Manali, India

Multiscale Lung Modeling 71 Viraj Shah