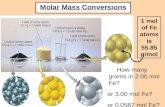
One liter of 5.0 M HCl contains how many moles of HCl? M = 5.0 mol= ? L = 1 L x = 5 mol.
-
Upload
audrey-gibson -
Category
Documents
-
view
218 -
download
1
Transcript of One liter of 5.0 M HCl contains how many moles of HCl? M = 5.0 mol= ? L = 1 L x = 5 mol.

Concentration

Molarity (M)
solutionofL
soluteofmoles
__
__
L
molM

One liter of 5.0 M HCl contains how many moles of HCl?
Practice
M = 5.0mol= ?L = 1 L
L
xM
10.5 x = 5 mol

55mL of 2.4M glucose contains how many moles of glucose?
Need to change mL to L
Practice
M = 2.4mol = ?L = 55mL 0.055L
L
xM
055.04.2 x = 0.132 mol

How could we make a 0.100M solution of CuSO4 * 5H2O?
Molar mass = 249.6 g/mol
Practice
L
xM
1100.0 x = 0.100 mol
mol
gmol
1
6.249100.0 24.96 g

Dilutions
2211 VMVM M is concentration in Molarity
V is volume (can be mL of L)

How can we make 500mL of 1.00M HCl from 6.00M HCl?
Practice
V1 = 500mLM1 = 1.00 MV2 = ?M2 = 6.00M
xMmLM 00.650000.1
x = 83.3 mL or 0.0833 L

What is the molarity of a 250mL solution containing 9.46g CsBr?
Molar mass of CsBr = 213 g/mol
Practice
gCsBr
molCsBrg213
146.9 0.0444 mol CsBr
L
molM
L
molCsBr
250.0
0444.0 0.176M

Percent by Mass
100_
_
solutiong
soluteg
** remember that solution is (solute + solvent)

What is the mass percent of a solution made from 199g of NiBr2 in 5.00x102 g of water?
Solution = solute + solvent
Practice
Solute = 199gSolution = 500g + 199g = 699g (solvent) (solute)
100699
199
g
g28.5% NiBr2